


Serbia's evolving landscape in the pharmaceutical sector has positioned it as a pivotal player in the EU drug approval harmonization process. This impressive acceleration in clinical research, coupled with a commitment to aligning local regulations with EU standards, not only enhances the country's attractiveness to international pharmaceutical companies but also significantly improves patient access to novel therapies. However, as Serbia navigates the complexities of this integration, critical questions arise:
This article delves into ten key insights that illuminate Serbia's crucial role in shaping the future of drug approvals within the European Union.
Bioaccess stands at the forefront of accelerating medical research in the region, leveraging its extensive knowledge of local regulatory frameworks to streamline the drug clearance process. With a robust network of healthcare experts and regulatory insight, Bioaccess significantly reduces the time required for EU drug approvals, often securing application approvals within an impressive 80 days. This efficiency is vital as Serbia's role in EU harmonization for drug approvals aligns its regulations with EU standards, making it increasingly attractive to pharmaceutical companies seeking swift market entry.
The nation has witnessed a remarkable surge in research studies, with global sponsors driving 84% of current investigations, particularly in oncology, gastroenterology, and neurology. Bioaccess's comprehensive services, including rapid site activation - over 50 sites activated in less than 8 weeks - and adherence to FDA/EMA/MDR compliance, ensure that innovative therapies reach patients faster than ever. As the healthcare system evolves, Bioaccess remains committed to facilitating rapid and compliant clinical trials, which underscores Serbia's role in EU harmonization for drug approvals within the global clinical research landscape.

The regulatory framework of the country is undergoing significant changes to align with EU standards, highlighting Serbia's role in EU harmonization for drug approvals, particularly through the implementation of the new Medicines Act. This legislation is designed to harmonize local regulations with EU directives, which supports Serbia's role in EU harmonization for drug approvals, enhancing transparency and efficiency in the drug assessment process. Consequently, Serbia's role in EU harmonization for drug approvals is essential for improving its regulatory environment, which is vital for attracting foreign investment in the pharmaceutical sector. This shift not only streamlines the approval process-allowing some research approvals to be finalized in as little as three weeks-but also facilitates quicker access to innovative therapies for patients.
Extensive research study management services, including:
are crucial in this transition. Regulatory specialists emphasize that these updates are essential for maintaining adherence to global best practices, ultimately fostering a more competitive environment for research in the region. The anticipated modifications in 2025 will further enhance submission procedures for research protocols, reinforcing the nation's commitment to ethical standards and participant safety in medical studies.
The integration of this country into the EU highlights Serbia's role in EU harmonization for drug approvals, offering significant opportunities for pharmaceutical companies while also presenting challenges in navigating complex regulatory requirements and ensuring compliance with new standards. Companies must adapt to these changes by investing in local expertise and fostering strong relationships with regulatory bodies.
By leveraging bioaccess's capabilities, which allow for obtaining regulatory consent in just 6-8 weeks and enrolling treatment-naive cardiology or neurology groups 50% faster than Western sites, companies can expedite their research studies. This strategic advantage not only enhances their ability to engage a diverse patient demographic but also improves recruitment initiatives, ultimately leading to successful clinical studies and quicker drug validations.
In this evolving Medtech landscape, collaboration is key. Companies that embrace these changes and utilize innovative solutions like bioaccess will be better positioned to navigate the complexities of clinical research and achieve their goals.

The nation's integration into EU regulatory frameworks has profoundly transformed drug authorization timelines, showcasing Serbia's role in EU harmonization for drug approvals and enabling the country to adopt best practices that enhance efficiency. Serbia's role in EU harmonization for drug approvals has notably reduced the time required for new medication authorizations by aligning its processes with EU standards.
For instance, research study applications are typically evaluated within a 60-day review period, with many approvals occurring even more swiftly, thanks to the effectiveness of the Medicines and Medical Devices Agency. This collaboration not only fosters trust among stakeholders, including pharmaceutical companies and healthcare providers, but also ultimately benefits patients by granting quicker access to innovative treatments.
Regulatory experts emphasize that Serbia's role in EU harmonization for drug approvals has established the country as a competitive player in the pharmaceutical sector. This commitment attracts foreign sponsors and enhances the overall environment for research trials. Furthermore, the establishment of a central ethics committee underscores the nation's resolve to uphold high standards in research, ensuring that ethical considerations are prioritized alongside regulatory compliance.
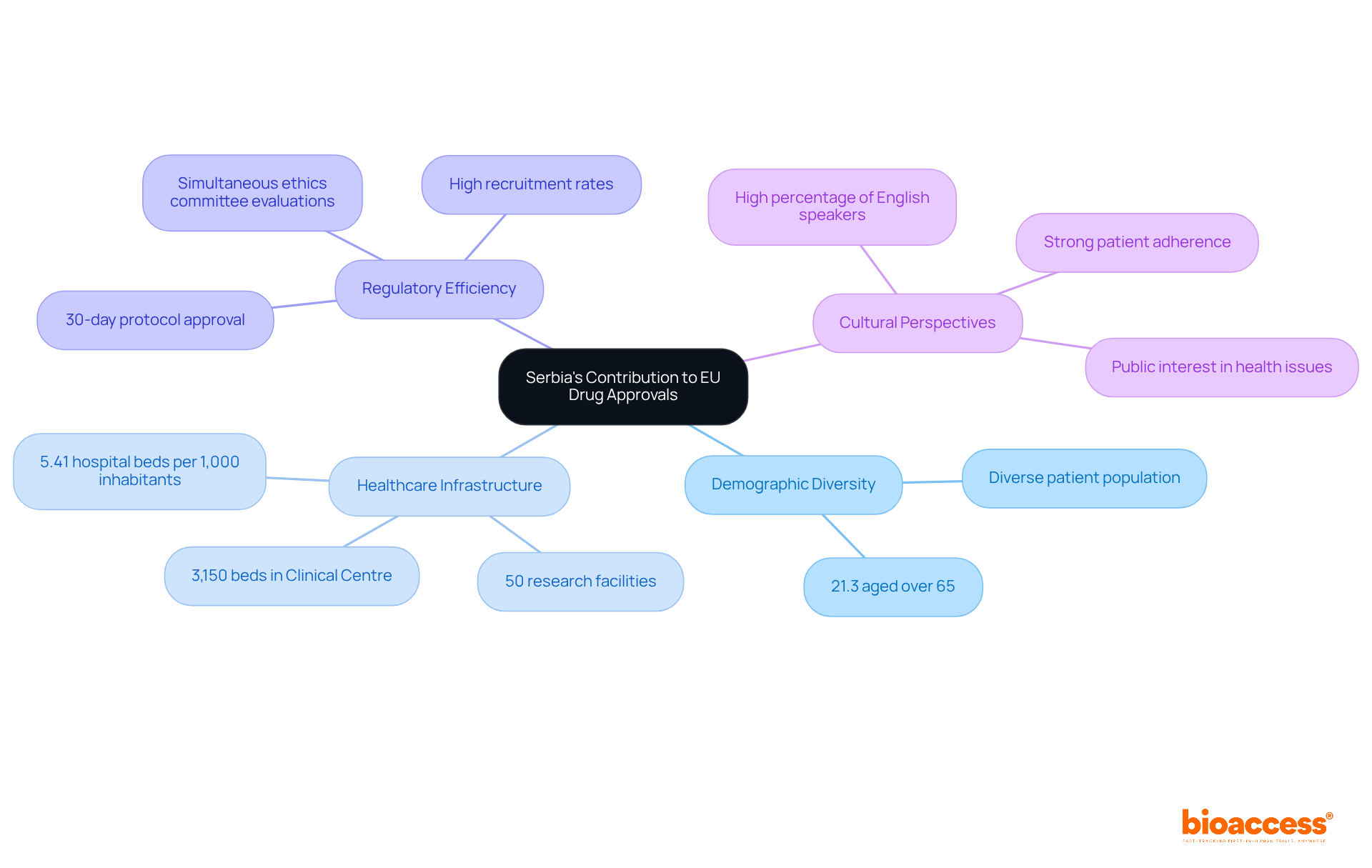
Local medical trials play a crucial role in strengthening Serbia's role in EU harmonization for drug approvals. With 21.3% of its residents aged over 65, the country boasts a diverse patient population, essential for studies targeting age-related conditions. This demographic diversity is further supported by a strong healthcare infrastructure, highlighted by the Clinical Centre of a certain Balkan nation, which houses over 50 research facilities and 3,150 beds, making it one of the largest medical institutions in Europe.
Research experts emphasize the effectiveness of the nation's patient recruitment strategies. A senior member of the regulatory affairs department noted that enrollment levels at Serbian sites significantly advanced global recruitment efforts, achieving patient targets ahead of schedule. This efficiency is bolstered by the country's regulatory structure, which allows protocols for medical studies to be sanctioned within 30 days, with some finalized in as little as three weeks.
The alignment of the nation's medical research regulations with EU standards highlights Serbia's role in EU harmonization for drug approvals, thereby enhancing its appeal to global sponsors. The efficient approval procedure, which can occur simultaneously with local ethics committee evaluations, ensures that studies can commence promptly. This has led to a notable increase in the number of research studies, particularly in oncology, where the country currently has 68 ongoing investigations.
Moreover, the cultural perspective on health issues in the region fosters strong patient adherence and eagerness to participate in research studies. This public interest, combined with reasonable research expenses and a significant proportion of English speakers, positions the country as a strategic site for pharmaceutical research. The varied patient demographic not only enriches the data collected during trials but also elevates the overall quality of clinical research, underscoring Serbia's role in EU harmonization for drug approvals as a vital participant in the drug regulation landscape.
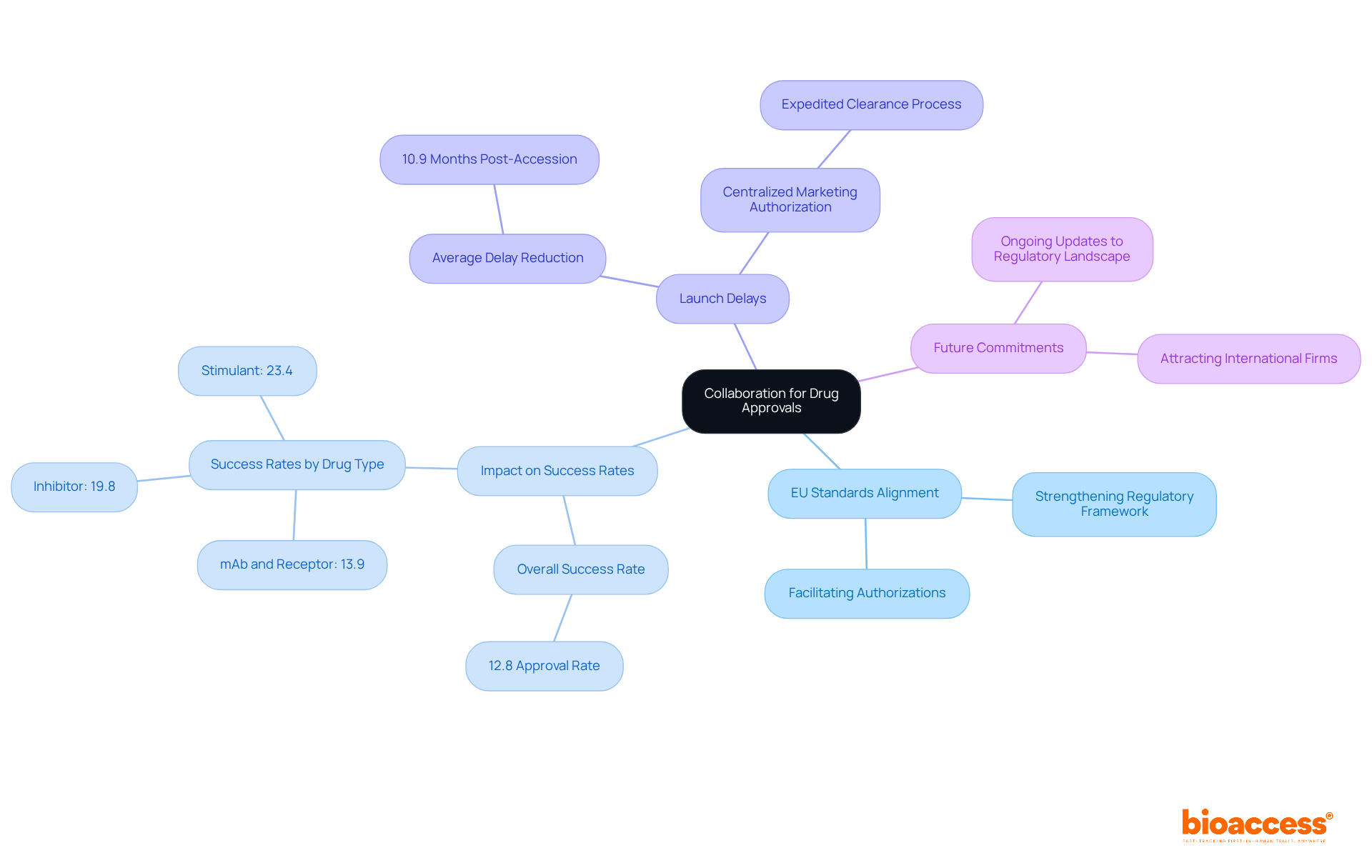
Collaboration between Serbian regulators and EU agencies is essential for highlighting Serbia's role in EU harmonization for drug approvals. By aligning their processes with EU standards, Serbian authorities strengthen Serbia's role in EU harmonization for drug approvals. This not only facilitates smoother authorizations but also bolsters the credibility of Serbia's regulatory framework. Notably, this alignment has been shown to positively impact drug authorization success rates, with recent studies revealing that the overall success rate for drug candidates stands at approximately 12.8%.
Countries that integrate EU practices experience a significant reduction in launch delays, averaging 10.9 months post-accession. Successful examples of this partnership include the adoption of centralized marketing authorization procedures, which have proven effective in expediting the clearance process for new pharmaceuticals. As we look ahead to 2025, ongoing updates reflect the nation's commitment to aligning its regulatory landscape with EU standards, further attracting international pharmaceutical firms and fostering a robust research environment.
Regulatory specialists emphasize that Serbia's role in EU harmonization for drug approvals is crucial for enhancing the overall efficiency and credibility of the medication assessment system. This collaboration not only addresses key challenges in clinical research but also positions Serbia as a competitive player in the global pharmaceutical landscape.
The execution of EU-compliant studies is significantly enhanced by Serbia's role in EU harmonization for drug approvals, making it a prime location for clinical research due to its diverse patient demographic. This variety ensures that study data accurately reflects different demographics, an essential aspect of Serbia's role in EU harmonization for drug approvals. By leveraging this diversity, researchers not only improve the validity of their results but also contribute to the development of treatments that are effective across various populations, ultimately leading to better patient outcomes.
For instance, Serbia has successfully conducted:
This impressive track record demonstrates the country's capability to address a wide range of health issues with diverse participant groups. Furthermore, the Clinical Centre of Belgrade stands out as a respected research hub, playing a crucial part in Serbia's role in EU harmonization for drug approvals by ensuring high-quality data collection and strict adherence to Good Clinical Practice (GCP) guidelines.
As medical researchers emphasize, including varied populations in studies is essential for comprehensively understanding treatment effectiveness and safety across different demographic groups. This approach supports the overarching goal of personalized medicine, which aims to tailor treatments to individual patient needs. In light of these insights, collaboration among researchers, healthcare providers, and regulatory bodies is vital to advancing clinical research and improving patient care.

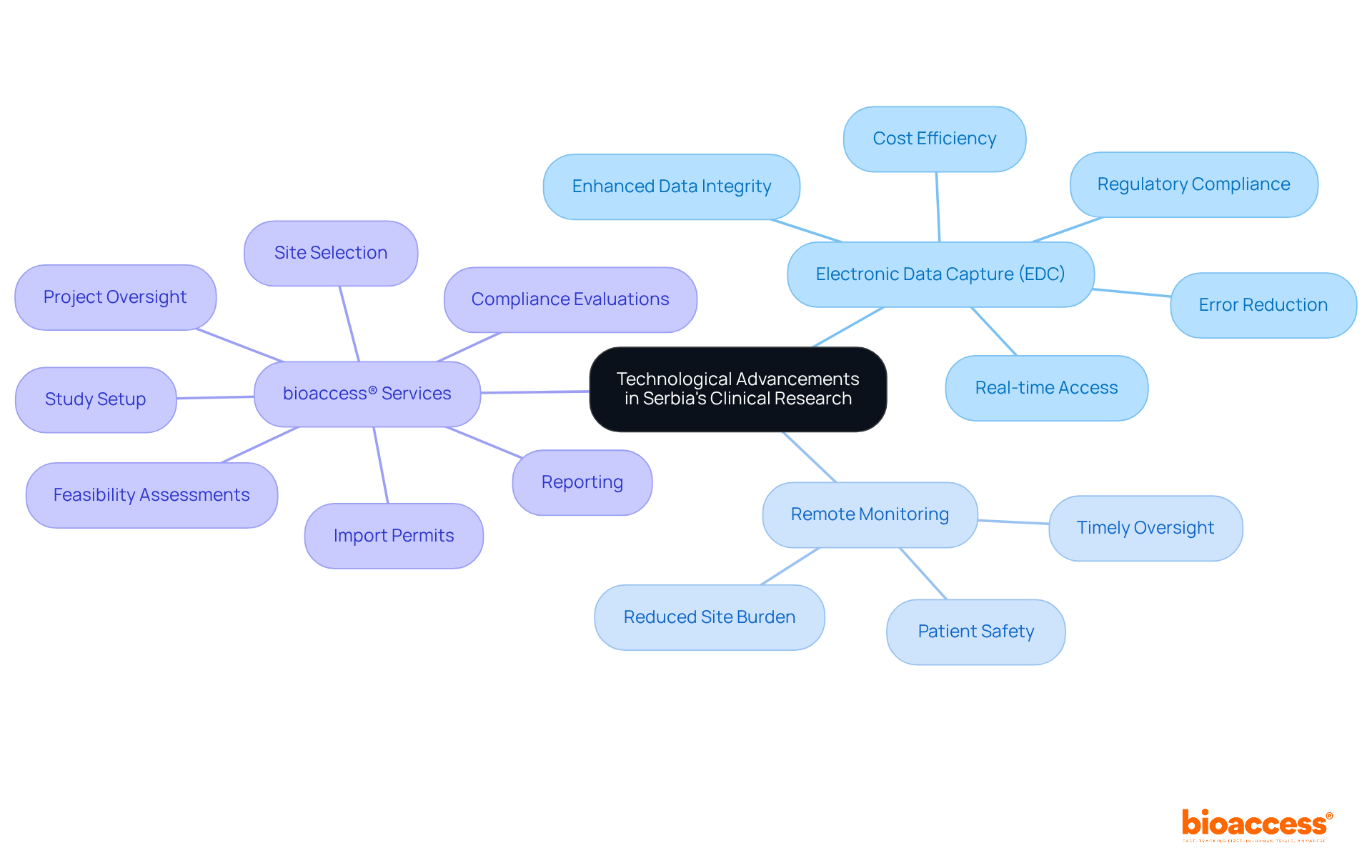
Technological advancements are reshaping Serbia's medical research landscape, underscoring Serbia's role in EU harmonization for drug approvals. Innovations such as electronic data capture (EDC) and remote monitoring are streamlining research processes, enhancing data quality, and ensuring compliance with regulatory standards. EDC systems automate data collection, significantly reducing reliance on manual input and minimizing errors, achieving error rates as low as 0.04%. This efficiency not only accelerates data management but also provides real-time access to critical information, empowering researchers to make swift, informed decisions.
The integration of remote monitoring technologies establishes live connections with clinical study sites, enabling timely oversight of patient safety and performance. This capability is vital for upholding high standards in trial management, particularly in light of Serbia's role in EU harmonization for drug approvals as it aligns its practices with EU regulations. Recent improvements in regulatory timelines have sparked optimism among biopharma firms, with Serbian studies now achieving patient enrollment rates that are 50% faster than traditional methods.
Industry leaders are increasingly recognizing the significance of these advancements. For example, bioaccess® emphasizes that leveraging EDC solutions enhances information management and expedites ethical approvals, resulting in substantial savings and improved outcomes. Furthermore, bioaccess® offers comprehensive research study management services, including:
These services collectively enhance the research process while ensuring adherence to EU regulations, which underscores Serbia's role in EU harmonization for drug approvals. As Serbia builds on its legacy of scientific innovation, the adoption of these technologies positions the nation as a competitive player in the global research arena, ultimately benefiting patient access to novel therapies. Clinical research directors should seriously consider integrating EDC and remote monitoring technologies into their studies to boost efficiency and compliance.
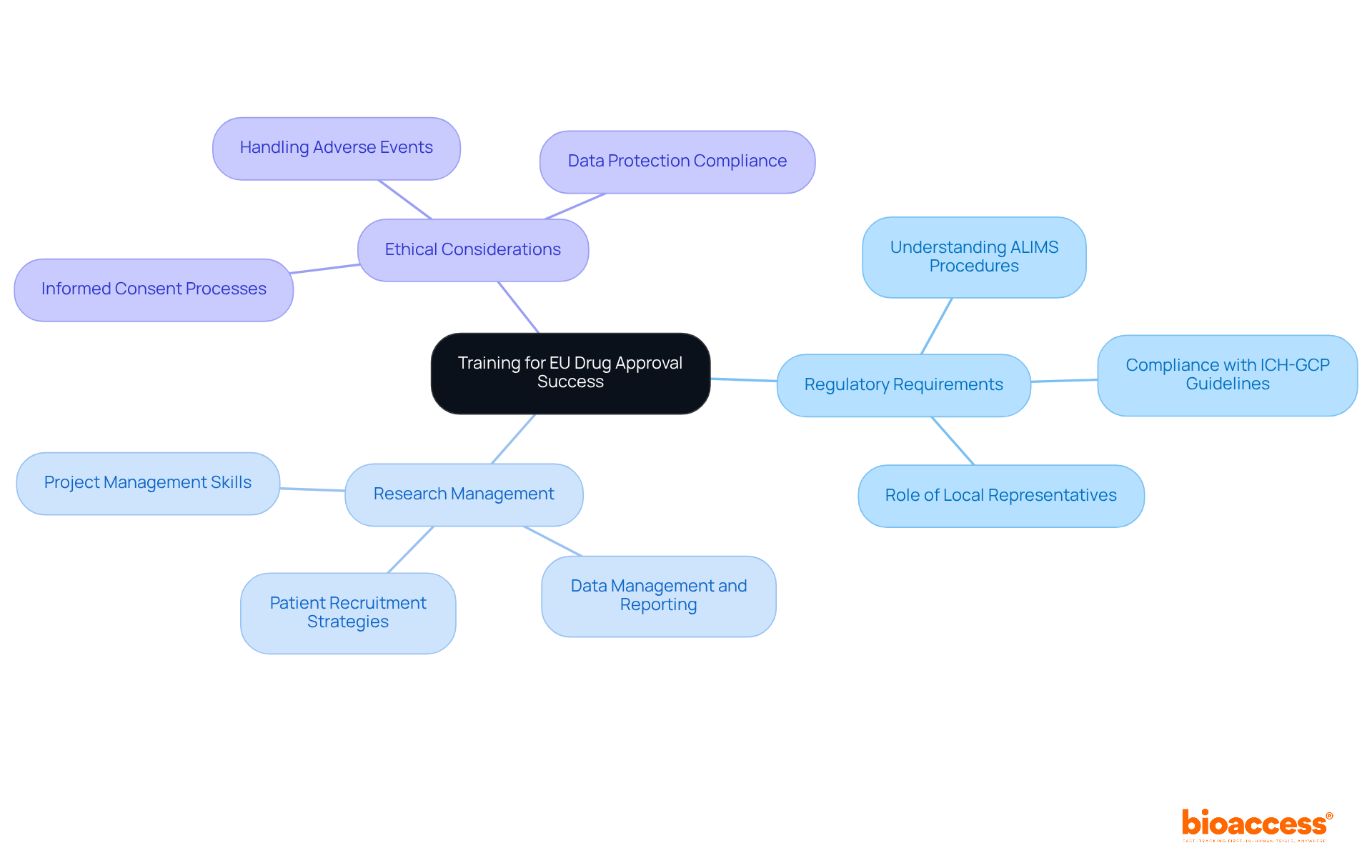
Educating research specialists in the region is crucial for achieving success in the EU drug approval procedure. This necessity underscores the importance of thorough training programs that encompass regulatory requirements, research management, and ethical considerations. Such initiatives are essential for cultivating a skilled workforce capable of navigating the complexities of medical research.
Investing strategically in human capital not only enhances the quality of clinical trials but also fortifies the region's competitive position within the European pharmaceutical market. Notably, successful workforce development initiatives have already begun to yield positive outcomes. Educational leaders are advocating for ongoing enhancements to training frameworks, emphasizing the need for continuous improvement.
By focusing on cultivating an informed and skilled workforce, the nation is poised to enhance Serbia's role in EU harmonization for drug approvals. This commitment to education and training is not just a response to current challenges; it is a proactive step towards ensuring long-term success in the industry.
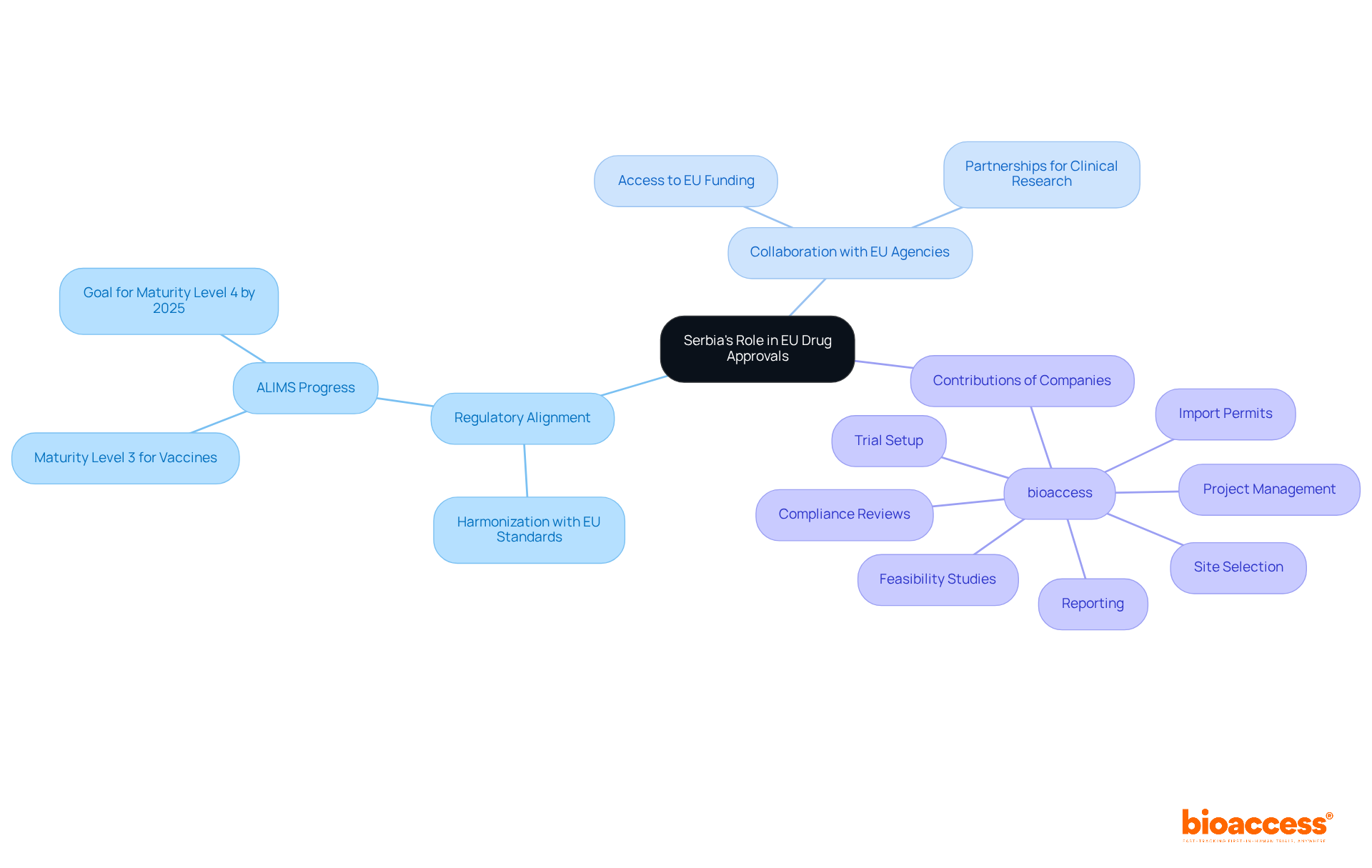
The future perspective for Serbia's role in EU harmonization for drug approvals appears promising. Ongoing efforts to align its regulatory framework with EU standards are crucial for understanding Serbia's role in EU harmonization for drug approvals. As the nation enhances its medical research capabilities and fosters collaboration with EU agencies, Serbia's role in EU harmonization for drug approvals positions it to become a significant player in the European pharmaceutical landscape.
Companies like bioaccess are pivotal in this transformation. They provide comprehensive clinical trial management services, including:
By leveraging its strengths - such as a diverse patient population and a growing pool of trained professionals - Serbia can attract more international pharmaceutical companies. This, in turn, facilitates faster access to innovative therapies for patients across Europe.
In summary, Serbia's role in EU harmonization for drug approvals is vital for advancing clinical research and improving patient outcomes in collaboration with EU agencies. The next steps involve continued alignment with EU standards and enhancing partnerships to solidify Serbia's role in EU harmonization for drug approvals within the European pharmaceutical market.
Serbia's pivotal role in harmonizing EU drug approvals is increasingly significant as the nation aligns its regulatory framework with European standards. This transformation streamlines the drug approval process and positions Serbia as a competitive player in the pharmaceutical landscape, attracting international investment and fostering innovation in clinical research.
The article outlines key insights into how Serbia enhances its contributions through various means. These include:
By leveraging its diverse patient population and fostering collaboration with EU agencies, Serbia is set to expedite access to groundbreaking therapies, ultimately benefiting both patients and pharmaceutical companies.
As Serbia evolves in its role regarding EU drug approvals, the emphasis on education and training for clinical research professionals becomes crucial. Stakeholders must embrace these developments and invest in local expertise to navigate the complexities of the regulatory landscape. The future outlook remains promising, with ongoing efforts likely to solidify Serbia's status as a key player in the European pharmaceutical market and enhance the overall efficiency of drug approvals across the region.
What is Bioaccess and its role in clinical research in Serbia?
Bioaccess is an organization that accelerates medical research in Serbia by leveraging its knowledge of local regulatory frameworks to streamline the drug clearance process. It significantly reduces the time required for EU drug approvals, often securing application approvals within 80 days.
How does Serbia's regulatory framework align with EU standards?
Serbia's regulatory framework is undergoing changes to align with EU standards, particularly through the implementation of the new Medicines Act. This legislation enhances transparency and efficiency in the drug assessment process, facilitating quicker access to innovative therapies for patients.
What are the key areas of research currently being conducted in Serbia?
The key areas of research in Serbia include oncology, gastroenterology, and neurology, with global sponsors driving 84% of current investigations.
What services does Bioaccess provide to facilitate clinical trials?
Bioaccess offers comprehensive services such as rapid site activation, feasibility analyses, site selection, compliance assessments, project management, and adherence to FDA/EMA/MDR compliance to ensure that innovative therapies reach patients faster.
What is the significance of the anticipated modifications in Serbia's regulatory processes by 2025?
The anticipated modifications in 2025 are aimed at enhancing submission procedures for research protocols, reinforcing the nation's commitment to ethical standards and participant safety in medical studies, and improving the overall regulatory environment.
What challenges do pharmaceutical companies face in Serbia's EU drug approval process?
Pharmaceutical companies face challenges in navigating complex regulatory requirements and ensuring compliance with new standards. They need to invest in local expertise and build strong relationships with regulatory bodies to adapt to these changes.
How can companies expedite their research studies in Serbia?
Companies can expedite their research studies by leveraging Bioaccess's capabilities, which allow for obtaining regulatory consent in just 6-8 weeks and enrolling treatment-naive cardiology or neurology groups 50% faster than in Western sites.
What is the importance of collaboration in the evolving Medtech landscape in Serbia?
Collaboration is crucial in the evolving Medtech landscape, as companies that embrace changes and utilize innovative solutions like Bioaccess will be better positioned to navigate the complexities of clinical research and achieve their goals.