


This article presents ten essential insights into zumab drugs, highlighting their development, mechanisms, therapeutic applications, and the challenges encountered in clinical research. Understanding the regulatory landscape is crucial, as is patient education and cost considerations, all of which play a significant role in enhancing clinical trial outcomes. For Clinical Research Directors, optimizing treatment development processes is paramount.
In the evolving Medtech landscape, bioaccess emerges as a pivotal player in addressing these challenges. By leveraging innovative strategies, stakeholders can navigate the complexities of drug development more effectively. This not only improves patient outcomes but also streamlines the research process, ensuring that new therapies reach those in need.
Collaboration among researchers, regulatory bodies, and healthcare providers is vital. As we move forward, it’s essential to foster partnerships that enhance knowledge sharing and resource allocation. The next steps involve a concerted effort to align objectives and drive progress in clinical research.
The landscape of clinical research is evolving at an unprecedented pace, especially with the emergence of innovative therapies such as zumab drugs. These monoclonal antibodies have transformed treatment protocols for a range of conditions, from cancer to autoimmune disorders. However, they also introduce unique challenges in both development and patient management. This article explores ten essential insights that Clinical Research Directors must consider to navigate the complexities of zumab drug research. By highlighting both the promising advancements and the critical considerations that can influence trial success, we aim to empower researchers. How can a deeper understanding of these nuances enhance outcomes in an increasingly competitive field?
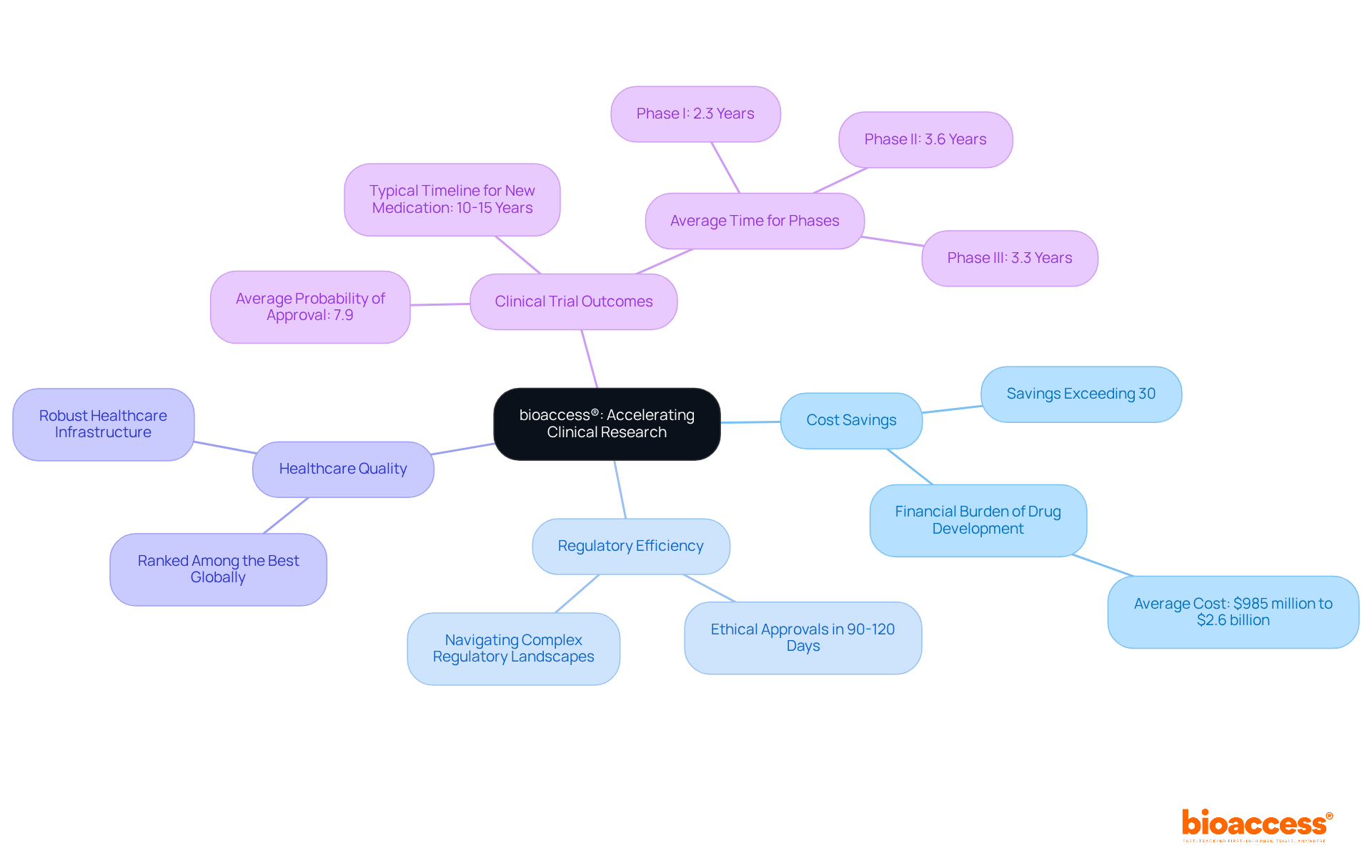
bioaccess® leverages its extensive expertise in early-phase clinical research to expedite the development of zumab drugs. By harnessing Colombia's competitive advantages—such as cost savings exceeding 30% compared to North America and Western Europe, regulatory efficiency with ethical approvals secured in just 90-120 days, and a high-quality healthcare system ranked among the best globally—bioaccess® guarantees swift site activation and patient recruitment. This rapid turnaround is essential for Clinical Research Directors striving to optimize treatment development processes and enhance patient enrollment rates.
With the typical timeline for creating a new medication ranging from 10 to 15 years, the flexibility offered by bioaccess® not only shortens these timelines but also boosts the likelihood of favorable clinical trial outcomes. Notably, the average probability of approval for substances in Phase I development stands at a mere 7.9%. The capability to navigate complex regulatory landscapes efficiently, bolstered by Colombia's robust healthcare infrastructure and R&D tax incentives, represents a significant advantage, enabling innovators to bring their therapies to market more swiftly and effectively.
The category of zumab drugs, which are monoclonal proteins, play a crucial role in modern medicine by precisely targeting antigens within the body. These engineered proteins bind to specific antigens, effectively blocking their action or tagging them for destruction by the immune system. This targeted mechanism not only enhances treatment efficacy but also minimizes potential side effects compared to traditional therapies. A key mechanism through which monoclonal proteins enhance immune response is dependent cellular cytotoxicity (ADCC), which recruits immune cells to eliminate targeted tumor cells.
For instance, Blinatumomab, a bispecific monoclonal protein, binds to CD19 on leukemia cells and CD3 on T cells, amplifying T cell activity against acute lymphocytic leukemia. This unique ability to bind to two different antigens is significant for its effectiveness in treating conditions like leukemia. Such real-world applications underscore the importance of understanding these mechanisms for Clinical Research Directors, as they directly influence trial design and patient selection criteria.
Moreover, ongoing research on zumab drugs seeks to improve the efficacy and safety of monoclonal treatments, indicating the evolving nature of this field. Insights from immunology specialists emphasize that this specificity enables these medications to function as precision instruments in oncology, targeting cancer cells while preserving healthy tissues. This precision is essential for creating effective treatment protocols.
Monoclonal proteins can be categorized into three types:
Understanding these categories is vital for navigating the complexities of clinical research and ensuring optimal patient outcomes.

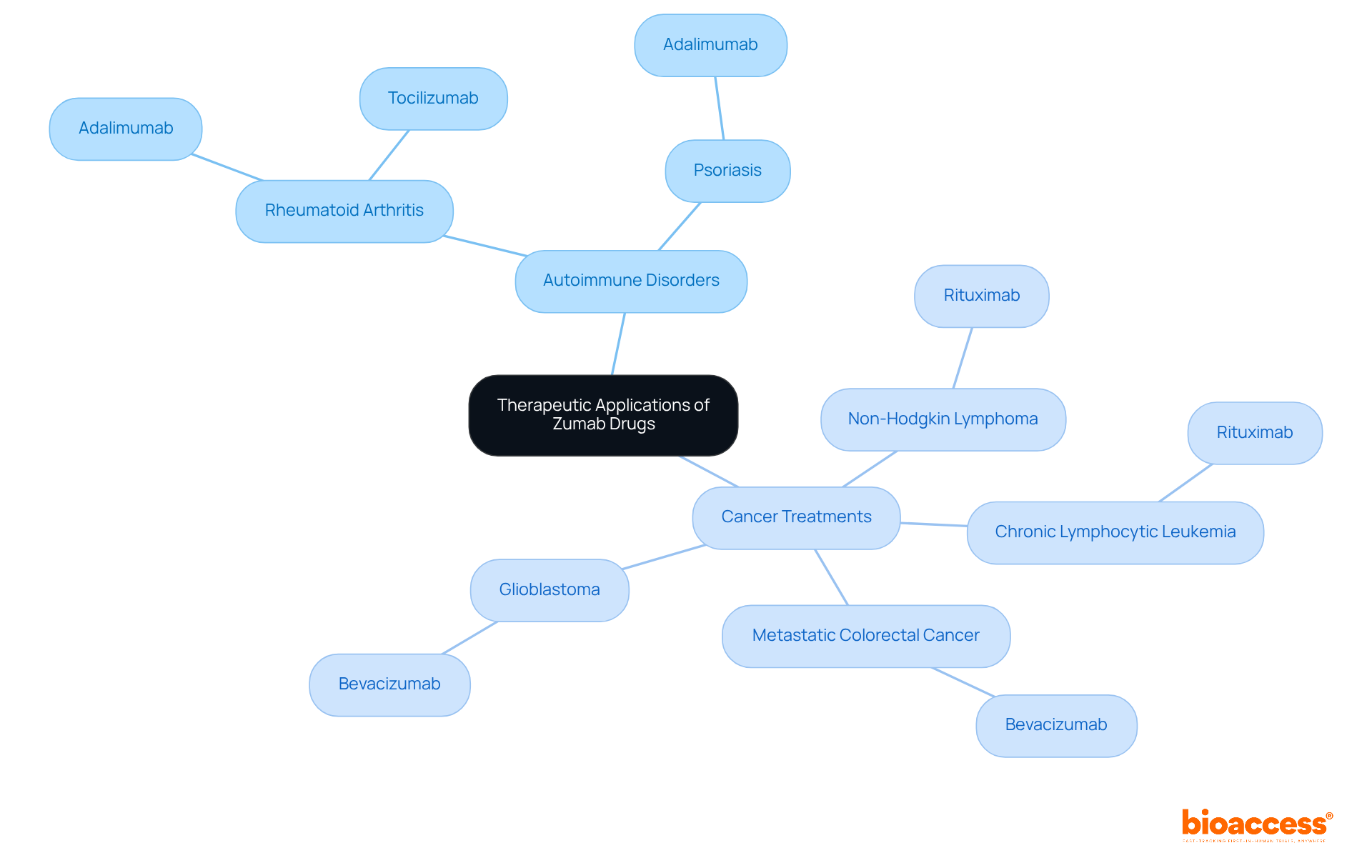
Monoclonal antibodies play a crucial role in addressing a range of conditions, particularly in cancer treatment and autoimmune disorders. Zumab drugs are frequently prescribed for:
Notably, Bevacizumab is utilized for multiple cancer types, such as metastatic colorectal cancer and glioblastoma, showcasing the extensive applications in oncology. The market for these therapies has seen substantial growth, reflecting their effectiveness and the increasing demand for targeted treatments.
For instance, Rituximab, a widely recognized medication, is employed for treating non-Hodgkin lymphoma and has demonstrated significant success in clinical studies, underscoring its potential to enhance patient outcomes. Additionally, Adalimumab is prescribed for various conditions, including rheumatoid arthritis and ulcerative colitis, highlighting the versatility of these therapies. Oncologists emphasize the importance of zumab drugs in managing complex conditions, highlighting their ability to specifically target cancer cells while minimizing damage to healthy tissue.
Understanding these applications enables Clinical Research Directors to strategically design clinical trials that focus on specific patient populations and therapeutic areas, ultimately advancing the development of effective treatments. However, it is essential to consider the serious negative effects associated with certain medications, including zumab drugs such as Denosumab and Rituximab, to provide a balanced view of their therapeutic applications.
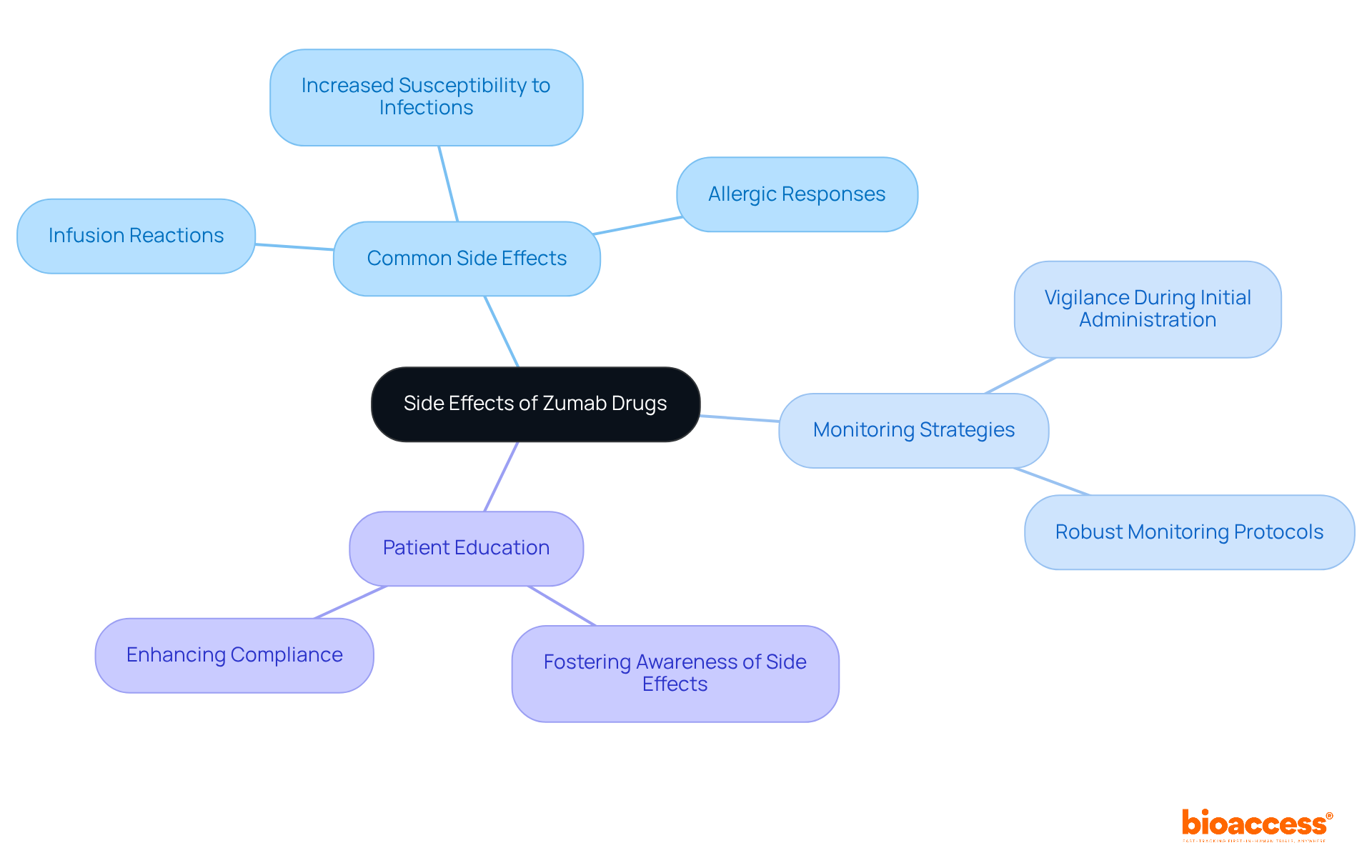
While generally well-tolerated, zumab drugs carry inherent risks that must be acknowledged. Common side effects include:
Notably, infusion-related reactions often arise during the initial administration, underscoring the necessity for vigilant monitoring protocols to ensure patient safety. Research indicates that hematological toxicity, such as neutropenia and lymphopenia, can heighten the risk of infections, particularly in patients undergoing concurrent chemotherapy. This highlights the critical need for Clinical Research Directors to implement robust monitoring strategies throughout the study process.
Educating patients about potential side effects is equally crucial. By fostering awareness, patients can more readily identify adverse reactions early on, significantly enhancing compliance and contributing to the overall success of clinical trials. Clinical researchers have observed that strict patient selection and clearly defined monitoring protocols are essential in mitigating risks associated with zumab drugs therapies. Practical examples, such as those documented in case studies on hematological toxicity linked to monoclonal therapies, demonstrate that proactive management of these risks can lead to improved patient outcomes and a more favorable safety profile for monoclonal treatments.
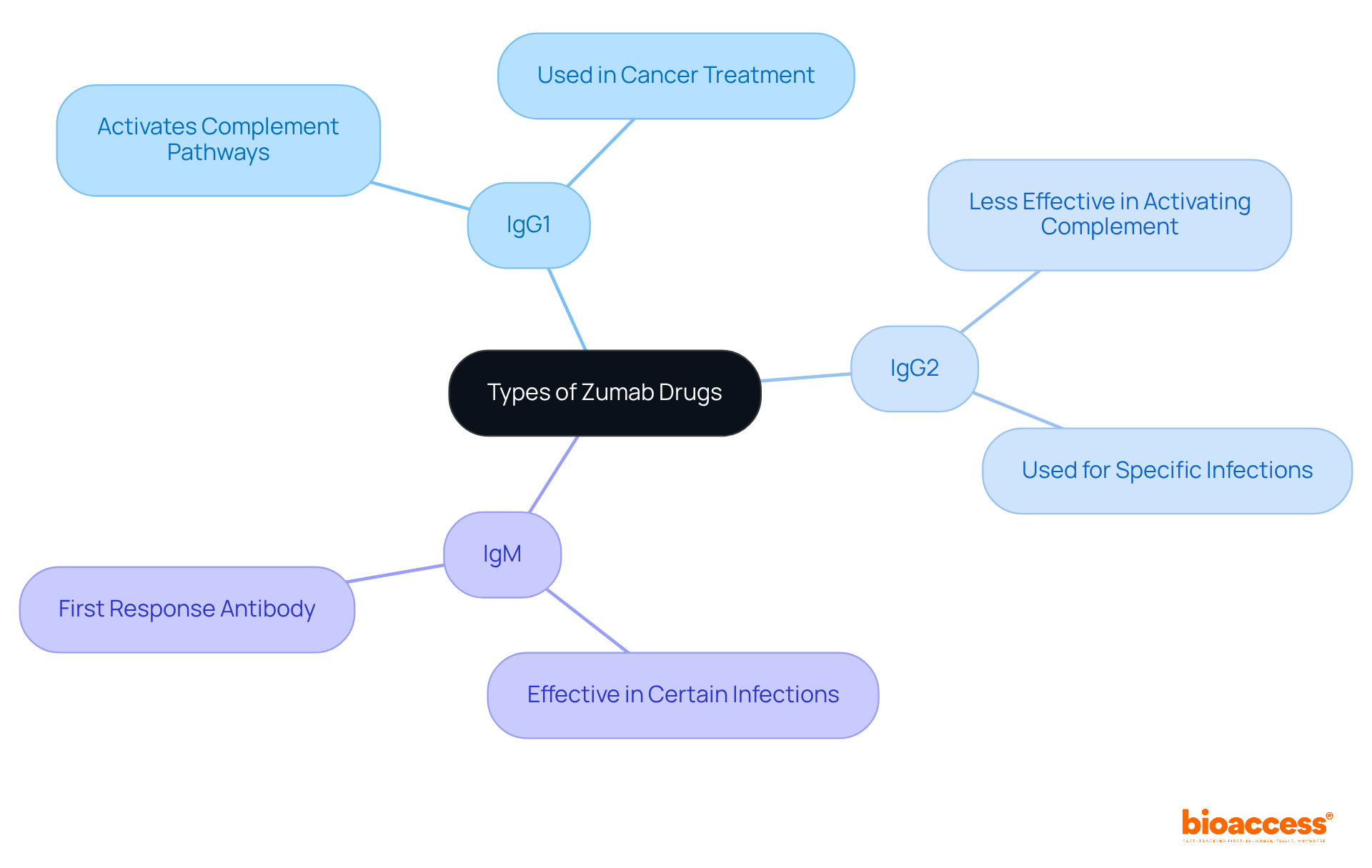
These medications can be classified into various categories, including IgG1, IgG2, and IgM monoclonal proteins, each with distinct characteristics and uses. For instance, zumab drugs like IgG1 Zumabs are often utilized for their ability to activate complement pathways, while other zumab drugs such as IgM Zumabs may prove more effective in certain types of infections.
Understanding these differences is crucial for Clinical Research Directors when designing studies and selecting the right therapeutic agents. This knowledge not only enhances study design but also ensures that the most appropriate therapeutic options are considered, ultimately leading to better patient outcomes.
Clinical studies play a pivotal role in assessing the safety and effectiveness of zumab drugs, which are classified as monoclonal antibodies. These trials unfold in a structured manner across several phases:
Phase I Studies: These initial studies involve a limited group of participants, focusing primarily on evaluating the safety and pharmacokinetics of the medication. Successful Phase I studies for zumab drugs typically demonstrate a favorable safety profile, which is crucial for advancing to subsequent phases.
Phase II Studies: Following positive Phase I results, Phase II studies expand the participant group to assess the medication's effectiveness while continuing to monitor safety. This phase is vital for determining the optimal dosing regimen and identifying any potential adverse effects.
Phase III Studies: These studies encompass larger populations and aim to confirm the medication's effectiveness, observe side effects, and compare it to standard therapies. Notably, the success rates for Phase I studies of monoclonal antibodies are significantly higher, with approximately 63%-70% of compounds progressing past this stage, which is essential for the overall development process.
Insights from clinical study experts underscore the importance of robust design that meets regulatory standards while addressing critical scientific questions. The transition from Phase I to Phase II is particularly significant, as only about 30%-40% of compounds successfully navigate this shift, highlighting the necessity for comprehensive preclinical data and strategic planning.
In conclusion, understanding the stages of clinical studies for specific medications, along with their success rates and expert insights, is vital for Clinical Research Directors aiming to effectively manage the complexities of medication development.
Navigating the regulatory landscape for zumab drugs is essential for Clinical Research Directors, as it demands a thorough understanding of the stringent requirements established by the FDA and EMA. To facilitate the approval process, these professionals must compile extensive documentation, including detailed clinical trial data, manufacturing processes, and comprehensive safety profiles. Recent statistics reveal that over 50 monoclonal agents have received FDA approval, underscoring a robust pipeline and the necessity of adhering to regulatory standards.
Moreover, the FDA's recent waiver of clinical efficacy studies for monoclonal antibody biosimilars is poised to reduce development costs by over 90% and accelerate approval timelines by more than 70%. This aligns with practices already adopted by the EMA, making it essential for companies to stay updated on these regulatory changes and guidelines. Real-world examples demonstrate how organizations have successfully navigated these requirements, highlighting the importance of strategic planning and thorough preparation in achieving timely approvals.
To enhance your chances of success in this complex environment, consider leveraging bioaccess's comprehensive services. These include:
All designed to support Medtech and biopharma startups in navigating the intricacies of regulatory compliance. Collaboration and proactive engagement with these services can significantly streamline your path to approval.
The future of zumab drugs development is indeed promising, fueled by ongoing research focused on enhancing specificity, minimizing side effects, and optimizing delivery methods. Innovations such as bispecific proteins, which can target multiple antigens simultaneously, are gaining traction due to their potential to significantly improve treatment efficacy. Clinical studies have demonstrated that these bispecific proteins can greatly enhance therapeutic outcomes in conditions like cancer and autoimmune disorders, with research indicating a notable increase in response rates.
Moreover, antibody-drug conjugates are emerging as a powerful tool, combining the targeting capabilities of these proteins with the cytotoxic effects of medications. This synergy enhances treatment accuracy while reducing systemic toxicity. As the antibody discovery market is projected to grow from USD 8.95 billion in 2025 to over USD 20.43 billion by 2034, at a CAGR of 9.54%, it is crucial for Clinical Research Directors to stay abreast of these developments. The increasing demand for zumab drugs necessitates the effective incorporation of new technologies and methodologies into clinical studies.
Patient education is vital in clinical research involving zumab drugs. By providing clear and accessible information about the drug's purpose, potential side effects, and the importance of adherence, Clinical Research Directors can significantly boost patient engagement and retention in studies. Research indicates that nearly 25% of clinical research participants do not complete their studies, often due to a lack of understanding or support. Moreover, dropout rates can soar between 25% and 70%, underscoring the urgent need for effective educational strategies.
Developing educational materials that address common concerns and questions is essential. These resources could include:
Additionally, creating an environment where patients feel comfortable voicing their worries can greatly enhance their overall experience. Strategies such as incorporating feedback from patient advocates highlight the importance of empowering patients with knowledge. For example, Joyce K. Anastasi emphasizes that inviting potential participants to share their questions and concerns is crucial for effective recruitment.
Furthermore, participants must feel competent and confident in using technology for successful engagement, as this significantly influences their willingness to participate. By prioritizing patient education and involvement, clinical studies can achieve better outcomes and ensure that participants feel informed and valued throughout their journey.
The development and administration of zumab drugs entail substantial costs, which include research, manufacturing, and patient care expenses. Clinical Research Directors must carefully assess these financial elements when planning studies, ensuring that budgets accommodate potential complications and secure sufficient funding. For instance, the average time from Investigational New Drug (IND) filing to Biological License Application (BLA) approval for First Tier monoclonal antibodies (mAbs) spans approximately six years, which can significantly impact study budgets.
Moreover, a cost-effectiveness assessment reveals that these medications offer considerable benefits over traditional therapies, particularly in terms of improved patient outcomes and reduced long-term healthcare costs. This insight is vital for shaping pricing strategies and enhancing market access considerations. The commercial success of mAbs often hinges on favorable reimbursement decisions and swift regulatory approvals.
By integrating these financial insights, Clinical Research Directors can navigate the complexities of budgeting for zumab drugs trials more effectively and optimize their overall research strategies. How can your organization leverage these insights to improve clinical research outcomes?
The development of zumab drugs marks a pivotal advancement in therapeutic options, especially for conditions like cancer and autoimmune disorders. By harnessing the unique properties of monoclonal antibodies, these drugs provide targeted treatment solutions that not only enhance efficacy but also minimize side effects. The insights shared in this article underscore the necessity of grasping the mechanisms, therapeutic applications, and regulatory considerations surrounding these innovative medications.
Key arguments presented include:
Moreover, the article highlights the significance of navigating the regulatory landscape and the future prospects of innovations in drug development, illustrating how these elements collectively impact the overall success of clinical research initiatives.
As the drug development landscape evolves, it is crucial for Clinical Research Directors to stay informed about these advancements and implement best practices in their research strategies. By cultivating a collaborative environment that prioritizes patient education and regulatory compliance, the potential for improving treatment outcomes and accelerating the approval process for new therapies can be greatly enhanced. Embracing these insights not only benefits clinical research but also ultimately leads to improved patient care and a promising future for medical innovations.
What is bioaccess® and how does it contribute to the development of zumab drugs?
bioaccess® is an organization that utilizes its expertise in early-phase clinical research to expedite the development of zumab drugs. It leverages Colombia's advantages, such as cost savings over 30%, quick regulatory approvals, and a high-quality healthcare system, to ensure swift site activation and patient recruitment.
What are the typical timelines for creating new medications, and how does bioaccess® affect these timelines?
The typical timeline for creating a new medication ranges from 10 to 15 years. bioaccess® shortens these timelines and increases the likelihood of favorable clinical trial outcomes, enhancing the efficiency of treatment development processes.
What is the average probability of approval for substances in Phase I development?
The average probability of approval for substances in Phase I development is approximately 7.9%.
What is the mechanism of action of zumab drugs?
Zumab drugs, which are monoclonal proteins, target specific antigens in the body by binding to them, blocking their action, or tagging them for destruction by the immune system. This targeted approach enhances treatment efficacy and minimizes potential side effects.
Can you provide an example of how a specific zumab drug works?
Blinatumomab is a bispecific monoclonal protein that binds to CD19 on leukemia cells and CD3 on T cells, enhancing T cell activity against acute lymphocytic leukemia. This dual binding capability is significant for its effectiveness in treating this condition.
What types of monoclonal proteins are there?
Monoclonal proteins can be categorized into three types: naked, conjugated, and bispecific.
What conditions are commonly treated with zumab drugs?
Zumab drugs are frequently prescribed for conditions such as rheumatoid arthritis, psoriasis, and various cancers including non-Hodgkin lymphoma and chronic lymphocytic leukemia.
What are some specific examples of zumab drugs and their applications?
Bevacizumab is used for multiple cancer types, including metastatic colorectal cancer and glioblastoma. Rituximab is used for non-Hodgkin lymphoma, while Adalimumab is prescribed for rheumatoid arthritis and ulcerative colitis.
What should Clinical Research Directors consider when designing clinical trials for zumab drugs?
Clinical Research Directors should consider the specific patient populations and therapeutic areas targeted by zumab drugs, as well as the serious negative effects associated with certain medications, to ensure balanced and effective trial designs.