


The article titled "10 Key Insights to Enhance Your PK Studies in Clinical Research" presents essential strategies and considerations aimed at improving pharmacokinetic (PK) studies within the realm of clinical research. It underscores the critical importance of:
These factors are not merely supplementary; they are foundational elements that significantly enhance the accuracy and effectiveness of PK studies. Ultimately, such enhancements lead to improved therapeutic outcomes and more streamlined research processes, reinforcing the necessity for ongoing advancements in this field.
The landscape of pharmacokinetic (PK) studies is rapidly evolving, propelled by advancements in technology and an enhanced understanding of drug behavior within the human body. The clinical research market is projected to reach nearly $70 billion by 2028, underscoring the critical importance of optimizing PK studies. This article delves into ten key insights that can significantly enhance the effectiveness of PK studies, ranging from innovative recruitment strategies to the integration of artificial intelligence in data analysis. How can researchers leverage these insights to navigate challenges and improve therapeutic outcomes in an increasingly competitive field?
bioaccess® leverages its extensive expertise in early-phase research to significantly accelerate drug absorption analyses for Medtech innovations. By capitalizing on Colombia's competitive advantages—such as cost reductions exceeding 30% compared to North America and Western Europe, regulatory efficiency with ethical approvals secured in an impressive 4-6 weeks, and a high-quality healthcare system recognized as one of the best globally—bioaccess® ensures effective patient recruitment and streamlined research timelines. This rapid turnaround not only facilitates quicker patient enrollment but also enables Medtech companies to expedite their product market entry, providing patients with faster access to groundbreaking therapies.
Industry leaders stress that swift regulatory processes are crucial for maintaining competitiveness in the evolving Medtech landscape, where the average time for ethical approvals worldwide often extends beyond several months. Successful examples of early-stage research demonstrate that harnessing these benefits can lead to substantial advancements in therapeutic development, particularly as trends in 2025 indicate a growing emphasis on adaptive research frameworks and real-time data application to enhance pk studies in pharmacokinetic evaluations. Furthermore, with the clinical research market projected to approach $70 billion USD by 2028, the increasing significance of efficient clinical research services is underscored.
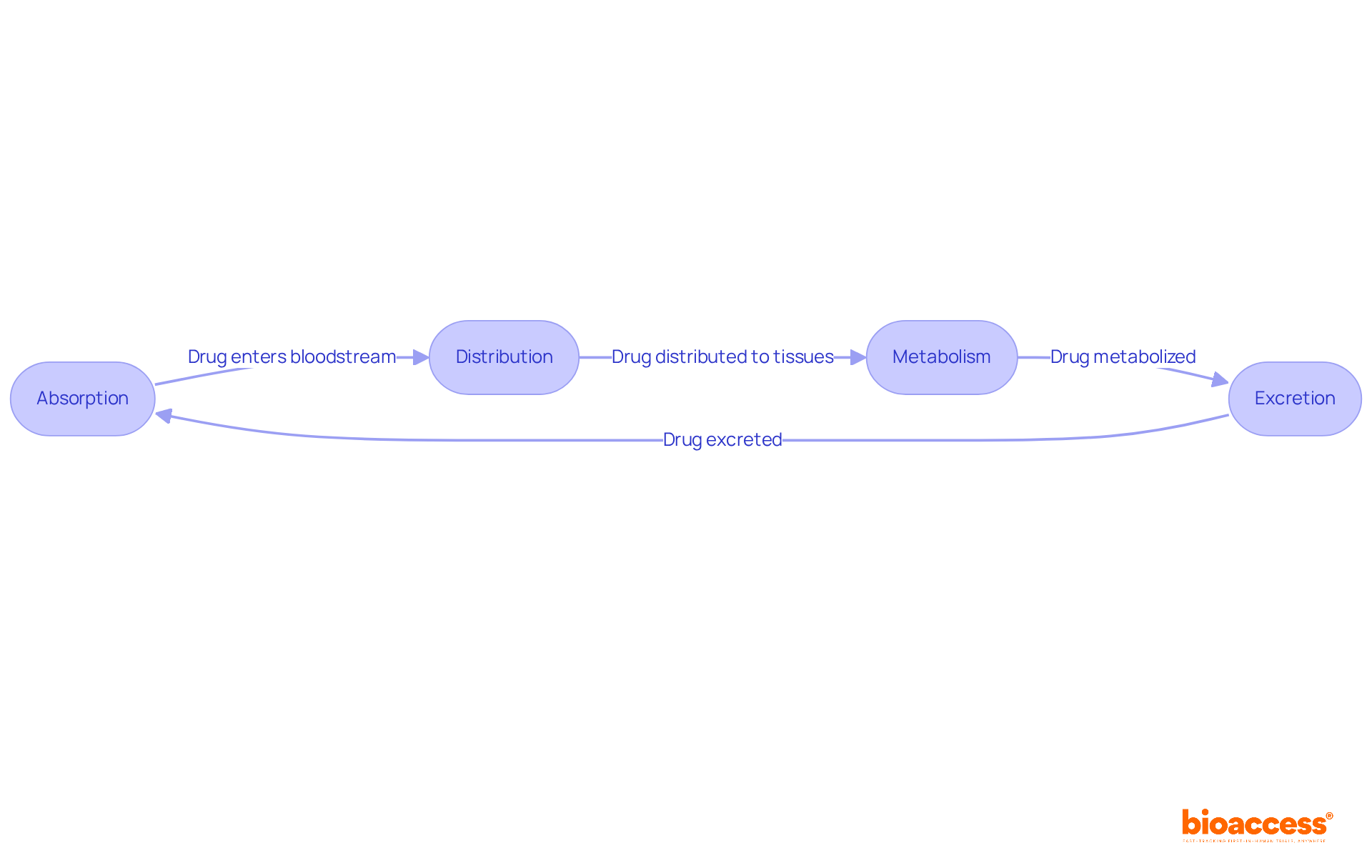
PK studies involve the examination of how substances move through the body, encompassing four key processes: absorption, distribution, metabolism, and excretion (ADME). Understanding these processes is crucial for predicting how a medication will act in the body, and PK studies directly influence dosing schedules and treatment efficacy. For instance, the rate of absorption can significantly impact the onset of action, while distribution plays a vital role in determining effectiveness at target sites. This knowledge not only informs clinical decisions but also enhances patient outcomes by ensuring that medications are utilized to their fullest potential.
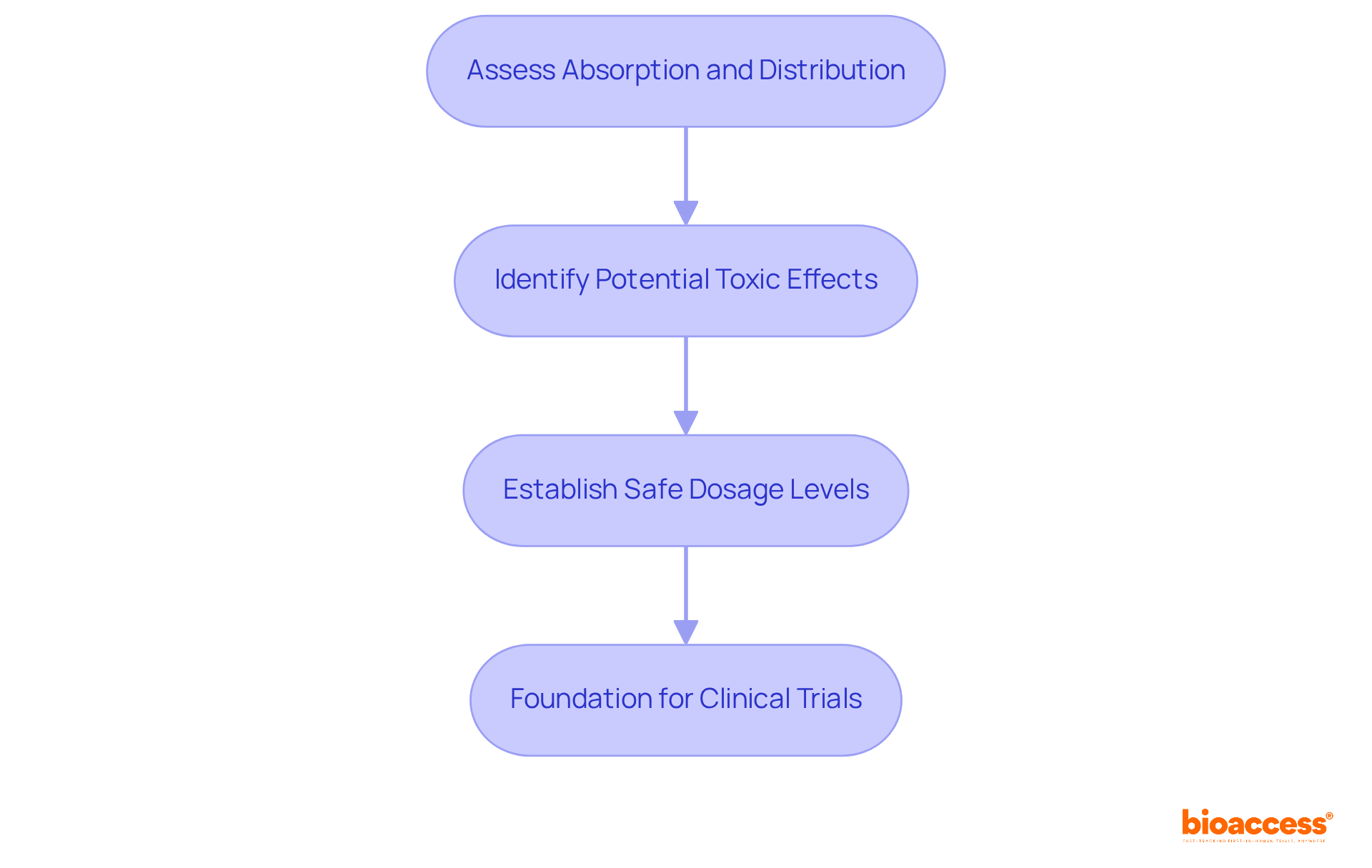
Preclinical assessments of medication behavior are crucial for determining a treatment's safety and effectiveness before entering clinical trials. These investigations typically encompass in vitro and in vivo experiments designed to evaluate how the medication is absorbed, distributed, metabolized, and excreted in animal models. Key actions involve:
This foundational understanding is essential in the Medtech landscape, as it addresses significant challenges in clinical research.
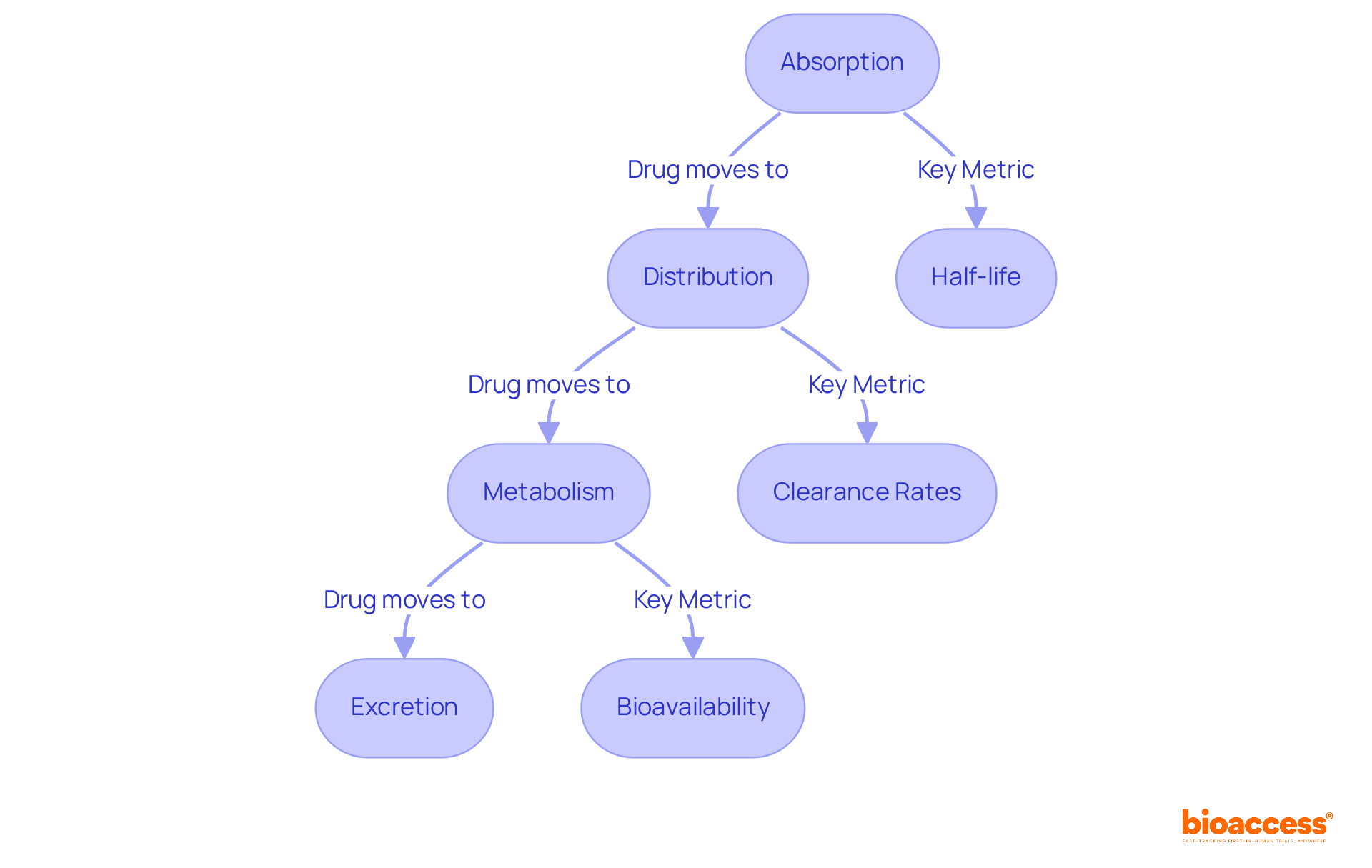
In vivo pk studies are pivotal for understanding medication behavior in living organisms, typically utilizing animal models to observe real-time absorption, distribution, metabolism, and excretion. These pk studies yield critical insights into a medication's therapeutic potential and safety profile, with meticulous measurements of factors such as half-life, clearance rates, and bioavailability.
For example, the average half-life of DPC1-RhB in animal models is less than six hours, indicating swift absorption and metabolism—information that is essential for informing dosing strategies in human trials. Recent findings from 2025 underscore the importance of these investigations, revealing that 83% of AstraZeneca's medication development projects progress to the trial phase without absorption issues. This highlights the necessity of integrating in vivo data into research.
Experts in the field assert that pk studies of real-time medication absorption analyses are vital for improving therapeutic outcomes, providing a robust foundation for understanding how substances interact within biological systems.
First-in-human (FIH) trials represent a crucial milestone in medical research. They are the initial pk studies conducted with human participants to assess the safety, tolerability, and pharmacokinetic properties of new medications. These pk studies are meticulously designed to gather essential data on how a drug interacts within the human body, focusing on its absorption, distribution, metabolism, and excretion (ADME). The insights gained from FIH research are pivotal in shaping subsequent trial phases and are indispensable for regulatory submissions.
Colombia presents significant advantages for conducting FIH studies. Notably, cost savings exceed 30% compared to trials in North America or Western Europe, and the regulatory review process typically spans only 90-120 days. The World Health Organization rates Colombia's healthcare system highly, and hospitals engaged in research must undergo a stringent ICH/GCP certification process. Furthermore, with a population exceeding 50 million and approximately 95% covered by universal healthcare, patient recruitment is robust. R&D tax incentives further enhance the attractiveness of Colombia, offering substantial financial benefits for investments in science and technology.
With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, you can ensure a comprehensive approach to clinical trial management. This approach effectively addresses the complexities of pk studies and pharmacokinetics analysis, reinforcing the importance of collaboration and the next steps in advancing clinical research.
Regulatory compliance is vital to pharmacokinetic research, ensuring strict adherence to established ethical and scientific standards. This process involves:
Such compliance not only safeguards the rights and welfare of participants but also bolsters the credibility of research findings. In 2025, ethical breaches in medical research continue to be a significant concern, with studies revealing that nearly 20% of trials encounter some form of ethical violation, highlighting the necessity for ongoing vigilance.
Experts emphasize that upholding ethical standards is paramount; as one regulatory authority remarked, 'Adhering to ethical guidelines is not just a regulatory requirement but a moral obligation to protect participants and ensure the integrity of the research process.'
Examples of ethical standards in pharmacokinetics include:
These are essential for fostering trust and accountability in clinical research.
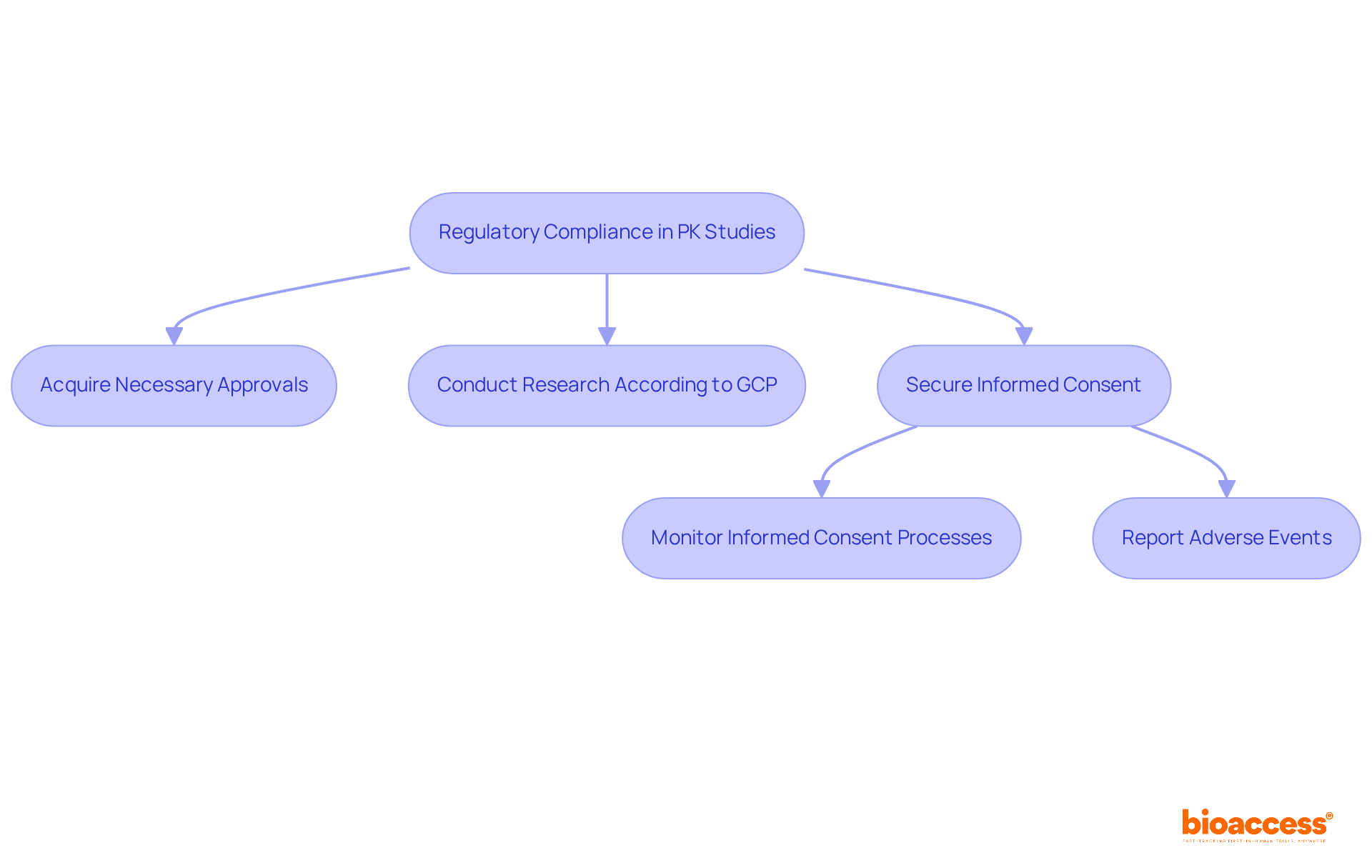
Bioanalytical techniques are essential for quantifying substance concentrations in biological samples, providing critical data for PK studies. Among these techniques, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is particularly notable for its unmatched sensitivity and specificity. Recent advancements in LC-MS/MS have demonstrated a remarkable accuracy rate, with research indicating that the method can achieve inter-assay precision with coefficients of variation (CVs) as low as 3%. This level of precision is crucial, as it ensures reliable PK data that directly impacts dosing regimens and therapeutic efficacy.
In 2025, innovative methods in bioanalysis have emerged, including the integration of hybrid immunocapture techniques with LC-MS/MS. This combination has achieved a linear dynamic range between 1-10 µg/mL, significantly enhancing the quantification of payloads even at trace levels. Such advancements facilitate a broader dynamic range in substance concentration measurement, with some studies indicating linear ranges from 0.2 to 100 µg/mL.
Insights from leading scientists highlight the critical role of LC-MS/MS in drug quantification. As Susan Pederson from Amgen Inc. states, "To assist nonclinical and clinical research, PK studies are necessary to evaluate the biosimilar and reference products with similar precision and accuracy." With the biosimilar market continuing to expand, the demand for robust bioanalytical techniques such as LC-MS/MS is poised to increase, solidifying its position as a fundamental component in the development and validation of drug metabolism assessments.
Attracting qualified researchers for PK studies presents significant challenges, including intense competition for talent, the necessity for specialized skills, and the complexities inherent in the design of these studies. Industry leaders have noted that research professionals in the medical field are in high demand, creating fierce competition among employers.
To effectively navigate these hurdles, organizations should implement targeted recruitment strategies. Leveraging professional networks can greatly enhance outreach, while offering competitive compensation packages is essential to draw top talent. Furthermore, providing opportunities for professional development fosters a culture of growth and aids in retaining skilled researchers.
As highlighted by AutoCruitment, "you can improve your targeting and recruitment efforts using innovative digital solutions provided by AutoCruitment." In 2025, the anticipated duration to hire qualified researchers for PK studies is approximately three to six months, underscoring the necessity for effective and strategic recruitment methods.
Moreover, with 80% of clinical trials postponed due to recruitment challenges, applying these strategies is critical for improving the likelihood of attracting the right talent to advance research in drug metabolism.
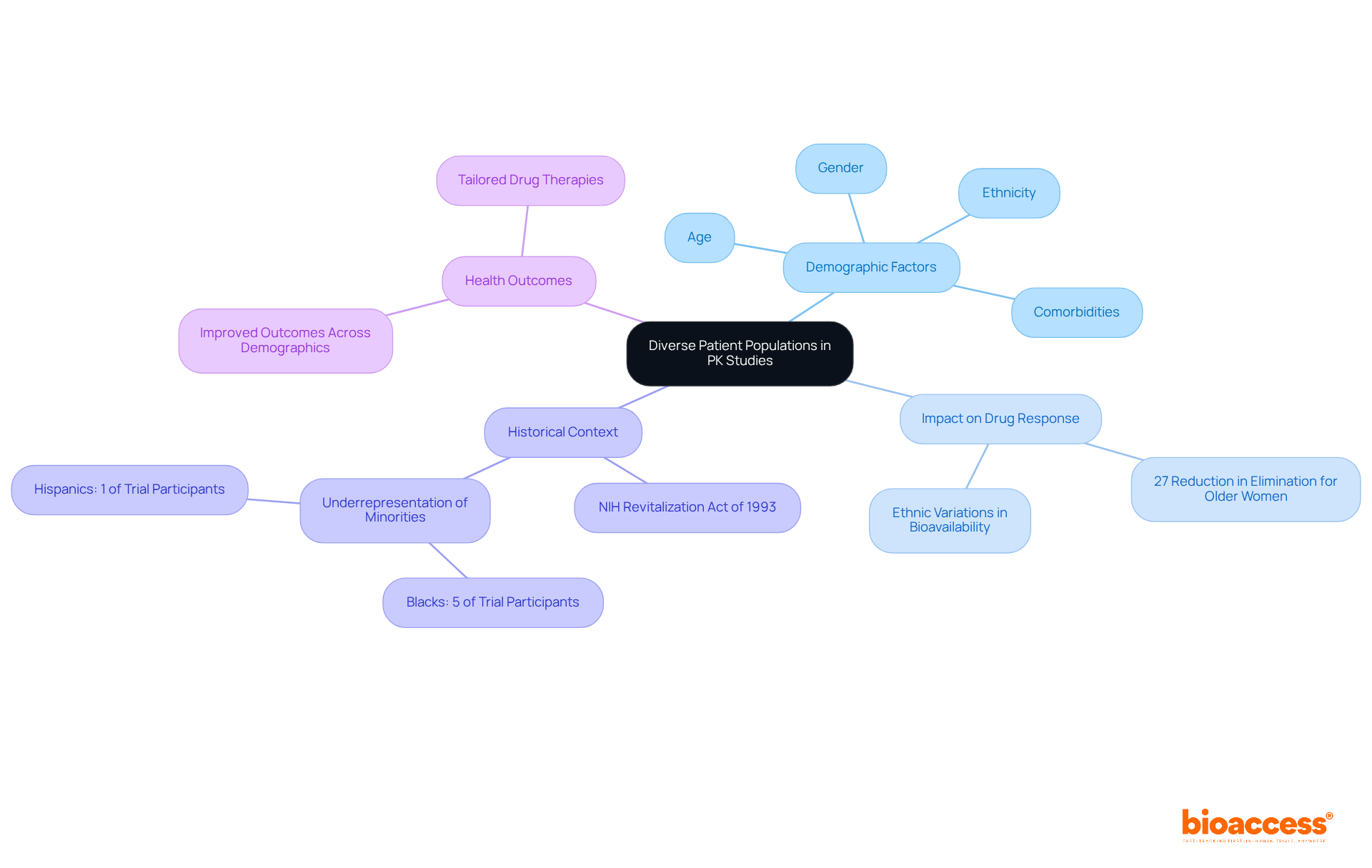
Including varied patient groups in pk studies is essential for acquiring representative information that accurately reflects the variability in responses across different demographics. Recent pk studies underscore that factors such as age, gender, ethnicity, and comorbidities can significantly influence pharmacokinetic parameters, including clearance rates and substance metabolism. For instance, research has shown that older women exhibit a 27% reduction in average elimination of certain medications compared to their younger counterparts, while ethnic variations can impact medication bioavailability and effectiveness.
As David W. Boulton emphasizes, it is crucial to determine whether diverse populations can safely and effectively utilize the medication at the recommended dose. By ensuring diversity in pk studies populations, researchers can enhance the validity of their findings, which leads to more effective and tailored drug therapies. This approach not only deepens the understanding of how various demographic groups respond to medications but also addresses the historical underrepresentation of specific populations in research studies. Notably, Blacks represent approximately 5% of trial participants despite accounting for 13% of the U.S. population.
Ultimately, this contributes to improved health outcomes across all demographics and aligns with ongoing initiatives, such as the NIH Revitalization Act of 1993, aimed at increasing the enrollment of women and minorities in clinical trials.
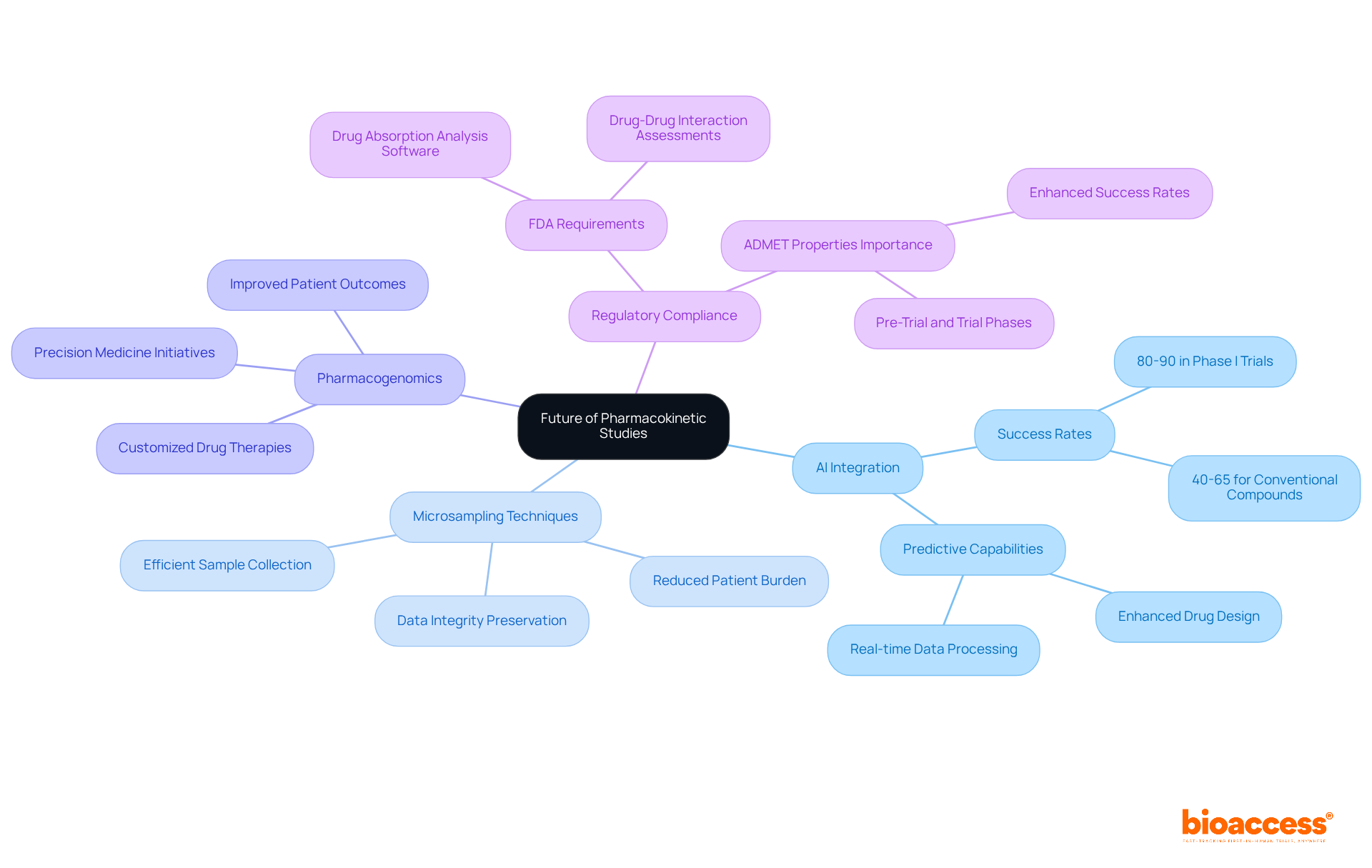
The future of pk studies is on the brink of a significant transformation, driven by cutting-edge technology and innovative research methodologies. The integration of artificial intelligence in data analysis stands out as a key trend, enhancing predictive capabilities and streamlining processes. For instance, AI-created medications are achieving success rates of 80-90% in Phase I trials, a remarkable improvement compared to the 40-65% success rates of conventionally developed compounds. Furthermore, the adoption of microsampling techniques facilitates more efficient sample collection, alleviating patient burden while preserving data integrity.
Pharmacogenomics is also gaining traction, enabling the customization of drug therapies based on individual genetic profiles. This approach not only enhances the precision of drug metabolism data but also supports personalized medicine initiatives, ultimately leading to improved patient outcomes. As the landscape of clinical research evolves, these innovations are set to redefine the standards of drug absorption studies, making them more precise and efficient than ever before. Thought leaders in the field emphasize that the integration of advanced technologies is crucial for optimizing medication development processes and enhancing therapeutic efficacy.
Moreover, the FDA's requirement for drug absorption and distribution analysis software to assess drug-drug interactions underscores the importance of regulatory compliance in this developing field. David B. Ascher notes that success rates could significantly improve if ADMET properties are adequately established during pre-trial and trial phases, highlighting the essential role of drug behavior analysis in medication development. The Deep-PK platform exemplifies how technology is advancing pk studies, providing robust predictive capabilities that enhance the overall efficiency of clinical trials.
The exploration of pharmacokinetic (PK) studies reveals critical insights that can significantly enhance clinical research and drug development processes. By understanding the dynamics of drug absorption, distribution, metabolism, and excretion, stakeholders can optimize treatment efficacy and patient outcomes. The advancements in methodologies, such as the integration of bioanalytical techniques and the emphasis on diverse patient populations, underscore the importance of a comprehensive approach to PK research.
Key arguments presented highlight the advantages of conducting PK studies in regions like Colombia, where regulatory efficiency and cost-effectiveness can accelerate the research timeline. Furthermore, the emphasis on ethical compliance and the incorporation of cutting-edge technologies, such as artificial intelligence and pharmacogenomics, are pivotal in shaping the future of pharmacokinetic studies. These innovations not only streamline processes but also enhance the precision of drug therapies tailored to individual patient needs.
As the clinical research landscape continues to evolve, embracing these insights and strategies is essential for advancing pharmacokinetic studies. The call to action is clear:
to ensure that the next generation of medications is both effective and accessible to all demographics. By doing so, the industry can drive forward the mission of improving health outcomes globally, ultimately transforming the way medications are developed and delivered.
What is bioaccess® and how does it benefit Medtech innovations?
bioaccess® is a research organization that accelerates drug absorption analyses for Medtech innovations by leveraging Colombia's advantages, such as significant cost reductions, efficient regulatory processes, and a high-quality healthcare system. This allows for quicker patient recruitment and streamlined research timelines, enabling faster market entry for Medtech products.
What are the competitive advantages of conducting research with bioaccess® in Colombia?
The competitive advantages include cost reductions exceeding 30% compared to North America and Western Europe, rapid ethical approvals secured in 4-6 weeks, and access to a high-quality healthcare system recognized globally.
Why are swift regulatory processes important in the Medtech landscape?
Swift regulatory processes are crucial for maintaining competitiveness as they allow for faster ethical approvals, which can take several months in other regions. This rapid turnaround helps Medtech companies expedite product development and market entry.
What are pharmacokinetics (PK) studies and why are they important?
PK studies involve examining how substances move through the body, including absorption, distribution, metabolism, and excretion (ADME). They are important for predicting medication behavior, influencing dosing schedules, and enhancing treatment efficacy, ultimately improving patient outcomes.
What are the essential steps in preclinical PK studies?
Essential steps in preclinical PK studies include assessing the absorption and distribution characteristics of a medication, identifying potential toxic effects, and establishing safe dosage levels for future human trials. These steps are crucial for determining a treatment's safety and effectiveness before entering clinical trials.
What is the projected growth of the clinical research market, and why is it significant?
The clinical research market is projected to approach $70 billion USD by 2028. This growth underscores the increasing significance of efficient clinical research services in the Medtech landscape, highlighting the need for rapid and effective research methodologies.