


The medical device industry presents a complex landscape, marked by critical phases that significantly influence the success of innovative products. As technology and healthcare continue to advance, grasping the key stages in the medical device life cycle is essential for stakeholders who aim to navigate this intricate process effectively. This article delves into ten pivotal phases, ranging from initial conceptualization to post-market surveillance, while highlighting the opportunities and challenges innovators encounter.
How can manufacturers ensure compliance and foster innovation amidst the ever-evolving demands of the healthcare environment?
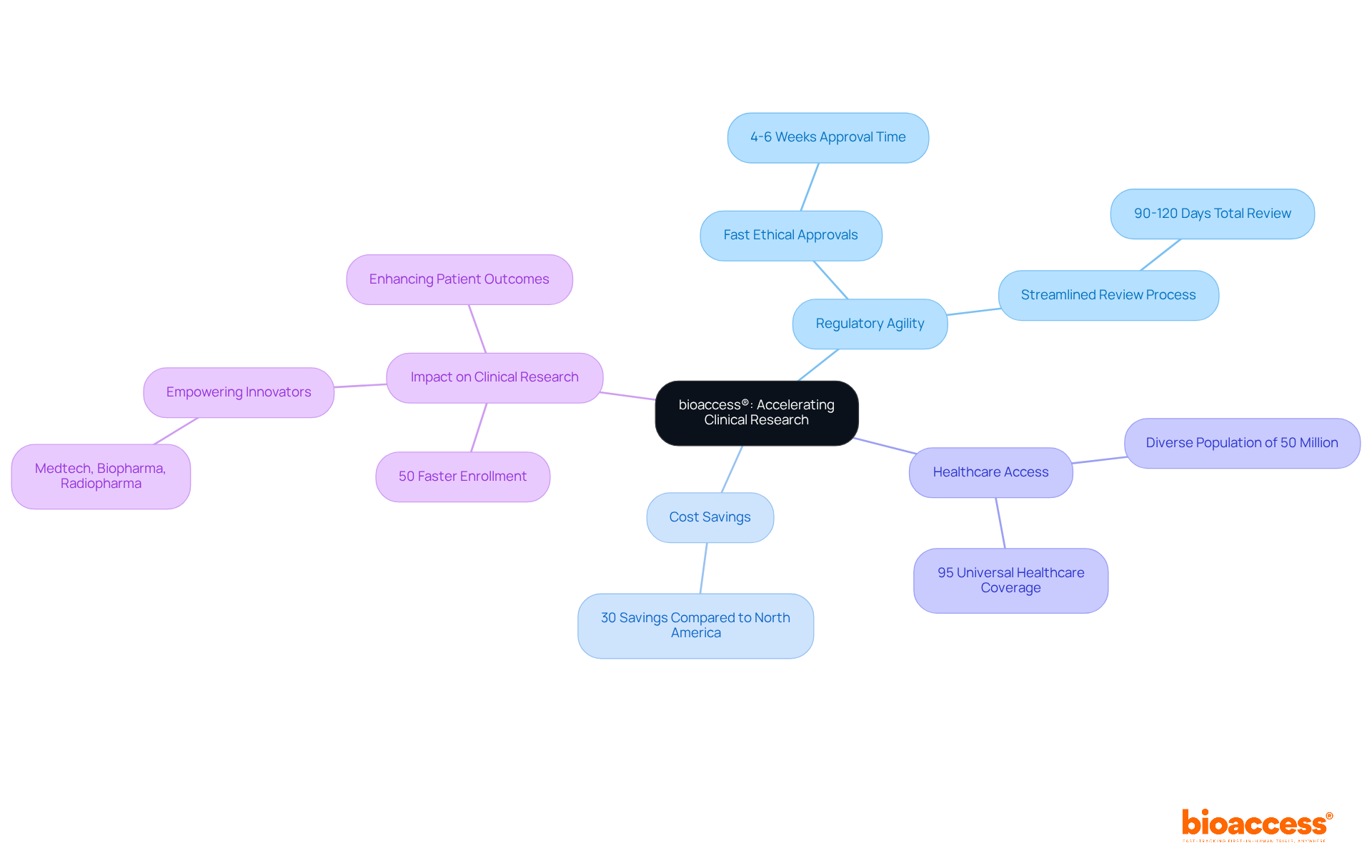
bioaccess® excels in expediting clinical research for medical devices, leveraging the regulatory agility of Latin America, particularly Colombia. This region offers significant cost savings of over 30% compared to North America and Western Europe. The total IRB/EC and MoH (INVIMA) review process in Colombia takes only 90-120 days, allowing for ethical approvals within an impressive 4-6 weeks.
Colombia's healthcare system ranks among the best globally, providing access to a diverse population of over 50 million, with 95% covered by universal healthcare. This strategic approach accelerates enrollment by 50% compared to traditional markets. By focusing on early-phase clinical research, bioaccess® empowers Medtech, Biopharma, and Radiopharma innovators to bring their products to market more swiftly.
This not only enhances patient outcomes but also drives advancements in medical technology. It underscores the critical role of regulatory speed and R&D tax incentives in the success of clinical trials. As you consider your own challenges in clinical research, think about how collaboration with bioaccess® can streamline your path to success.
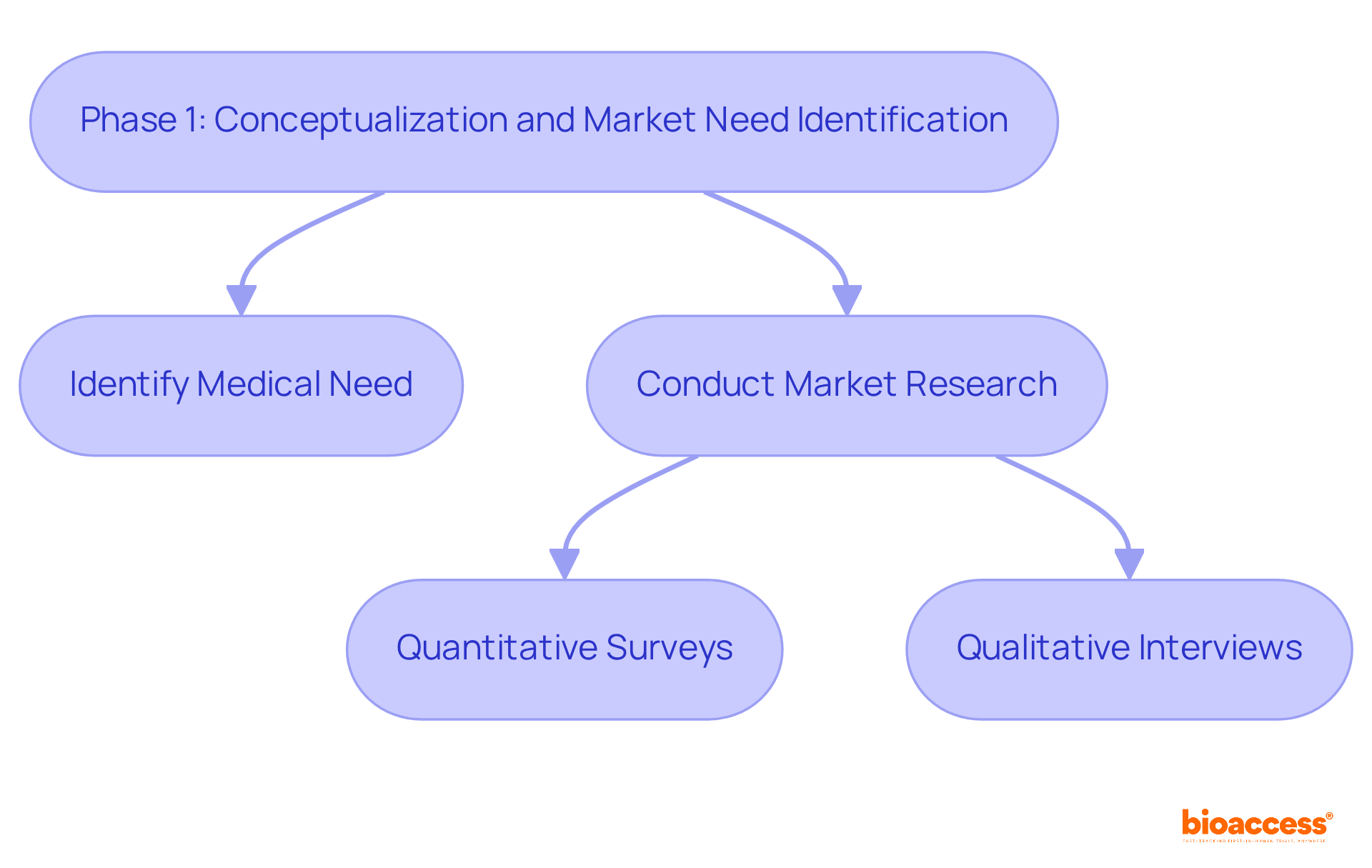
The initial phase focuses on identifying a specific medical need and crafting a solution that effectively bridges this gap. Thorough market research is essential to understand existing solutions and their limitations, especially considering that approximately 30% of medical products fail due to unmet market demands. Engaging with healthcare professionals and potential users is crucial; their insights can shed light on practical challenges faced in clinical environments. This phase is pivotal, as it shapes the design and functionality of the device, ensuring alignment with the needs of both users and healthcare providers.
Successful market research strategies encompass:
These strategies gather diverse perspectives. This approach ultimately steers the development process toward a solution that enhances patient care and outcomes. By prioritizing collaboration and leveraging expert insights, we can address key challenges in the Medtech landscape effectively.
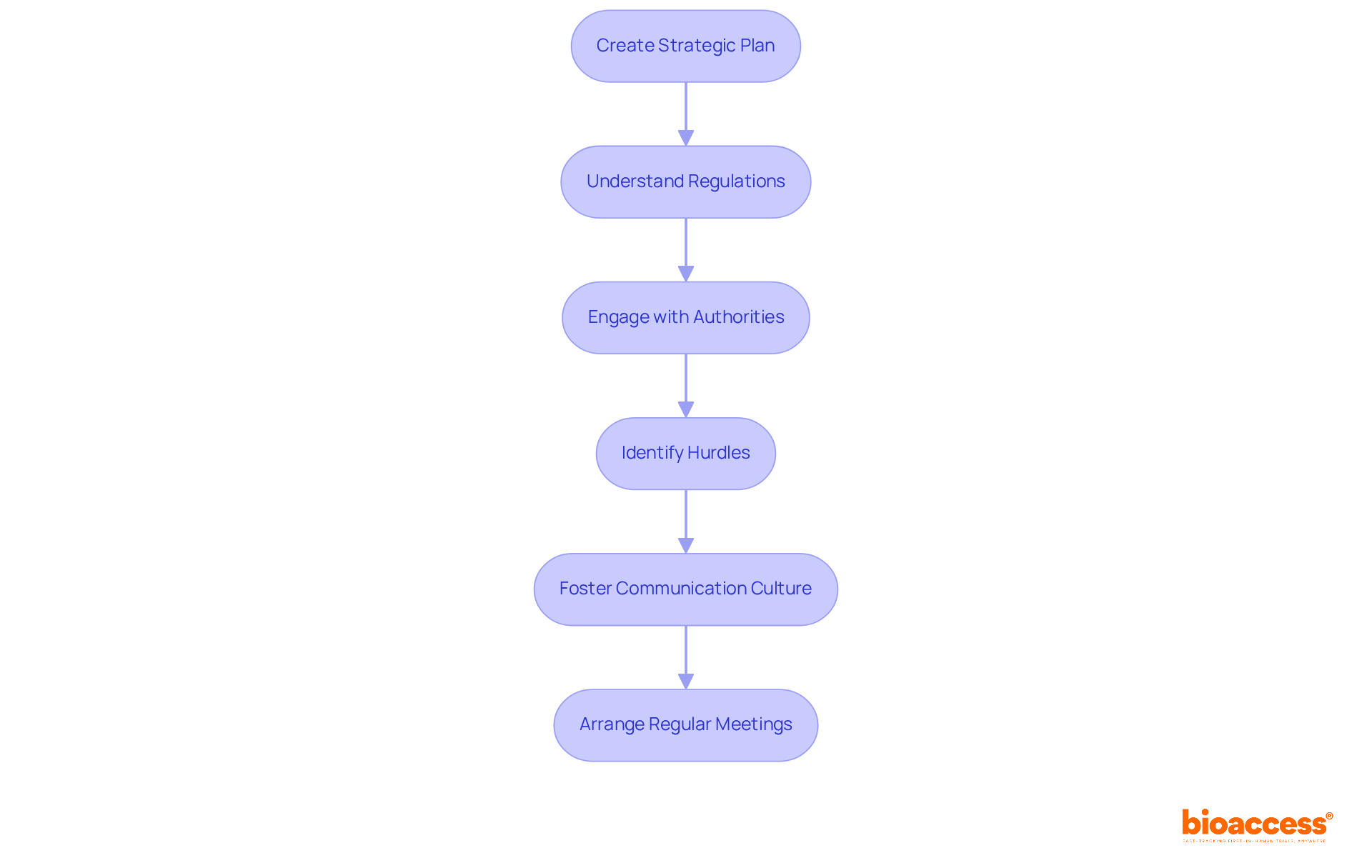
In this phase, developers must create a comprehensive strategic plan that outlines the development timeline, budget, and compliance pathway. A clear understanding of the specific regulations relevant to the apparatus is essential, as these regulations significantly influence design decisions and testing protocols. Interacting with governing entities early in the process not only facilitates smoother approvals but also helps identify potential hurdles. This proactive approach minimizes delays and ensures that the device is designed with compliance at the forefront.
Statistics reveal that 70% of costly compliance issues stem from communication breakdowns rather than complexity. This underscores the critical need for clear dialogue with authorities. As the oversight environment evolves in 2025, with increased scrutiny and a focus on real-world evidence, remaining knowledgeable and adaptable is vital for successful product development. Experts emphasize that fostering a culture of adherence within organizations can greatly enhance communication with oversight authorities, ultimately leading to more effective approval processes.
For instance, engaging with local and national organizations can provide valuable insights into policy changes and best practices. Consequently, it is crucial for developers to not only grasp the regulations but also to actively participate in discussions that shape the governing landscape. Additionally, leveraging services like those offered by bioaccess - such as trial set-up, start-up, approval, and reporting - can streamline this process, ensuring that clinical trials are conducted efficiently and effectively.
As a practical suggestion, developers should arrange regular meetings with oversight entities to stay informed on changes and promote strong communication. This collaborative approach not only enhances compliance but also positions developers for success in navigating the complexities of clinical research.
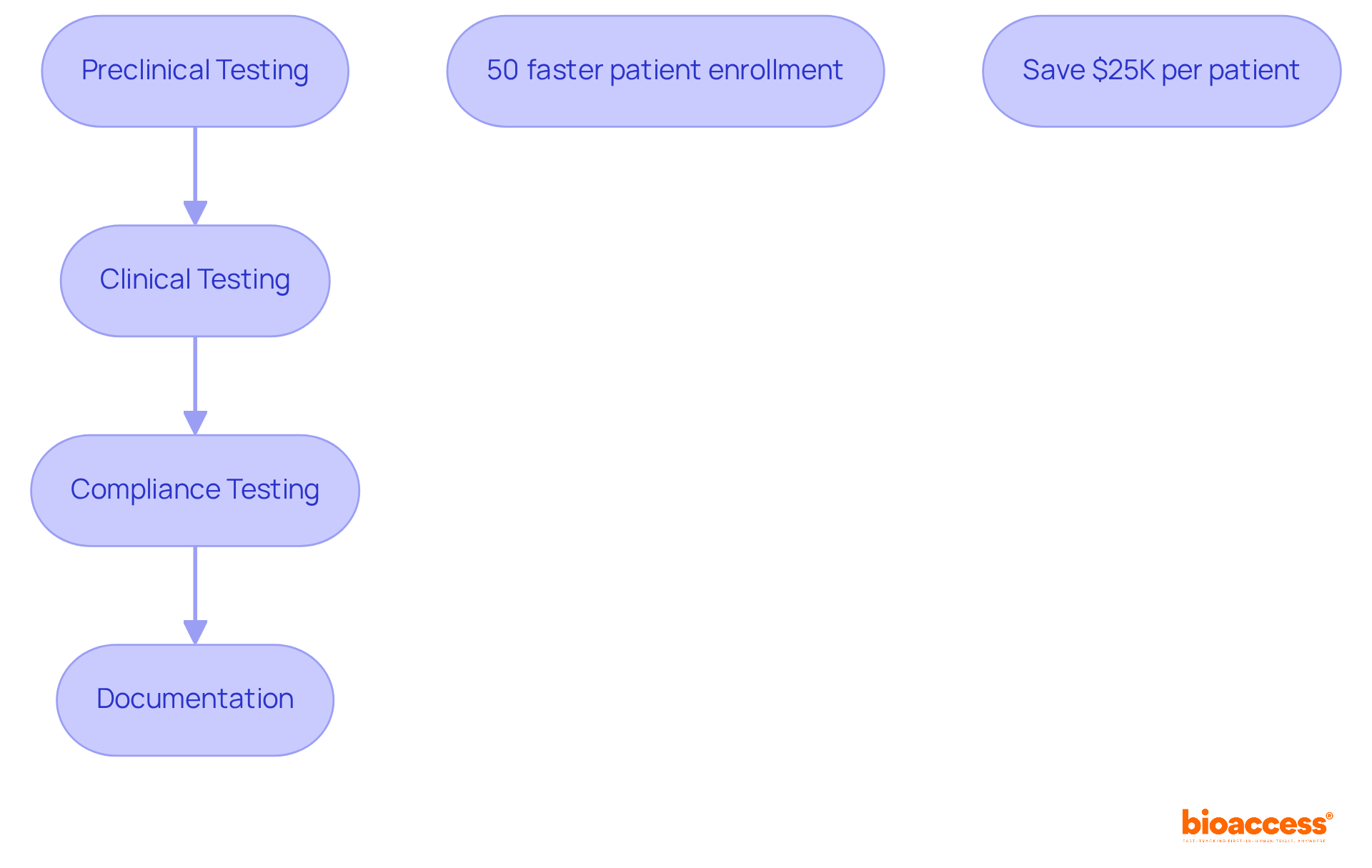
Validation stands as a crucial stage in the life cycle of medical devices, requiring rigorous testing to ensure that products perform as intended and comply with all regulatory requirements. This process encompasses both preclinical and clinical testing phases, where equipment undergoes meticulous evaluation for safety, effectiveness, and usability. Compliance testing is essential for confirming adherence to established standards and regulations, a vital step for securing market approval. Typically, the duration for compliance testing spans several months, reflecting the complexity and thoroughness required in the evaluation process. Thorough documentation of these testing processes is imperative, as it provides the necessary evidence to demonstrate compliance to regulatory authorities.
Incorporating bioaccess®'s capabilities can significantly streamline the validation and compliance testing phases. With bioaccess®, clinical trials can achieve 50% faster patient enrollment and save $25K per patient, thanks to FDA-ready data that eliminates rework and delays. This efficiency not only accelerates the validation process but also enhances strategies for managing the life cycle of medical devices.
As Richard P. Feynman aptly noted, 'The first principle is that you must not fool yourself - and you are the easiest person to fool.' This underscores the importance of maintaining integrity and rigor throughout the testing phases. Furthermore, insights from clinical researchers emphasize that 'Research is formalized curiosity. It is poking and prying with a purpose,' as stated by Zora Neale Hurston. This reinforces the necessity for a systematic approach to validation and compliance testing in the medical equipment industry.
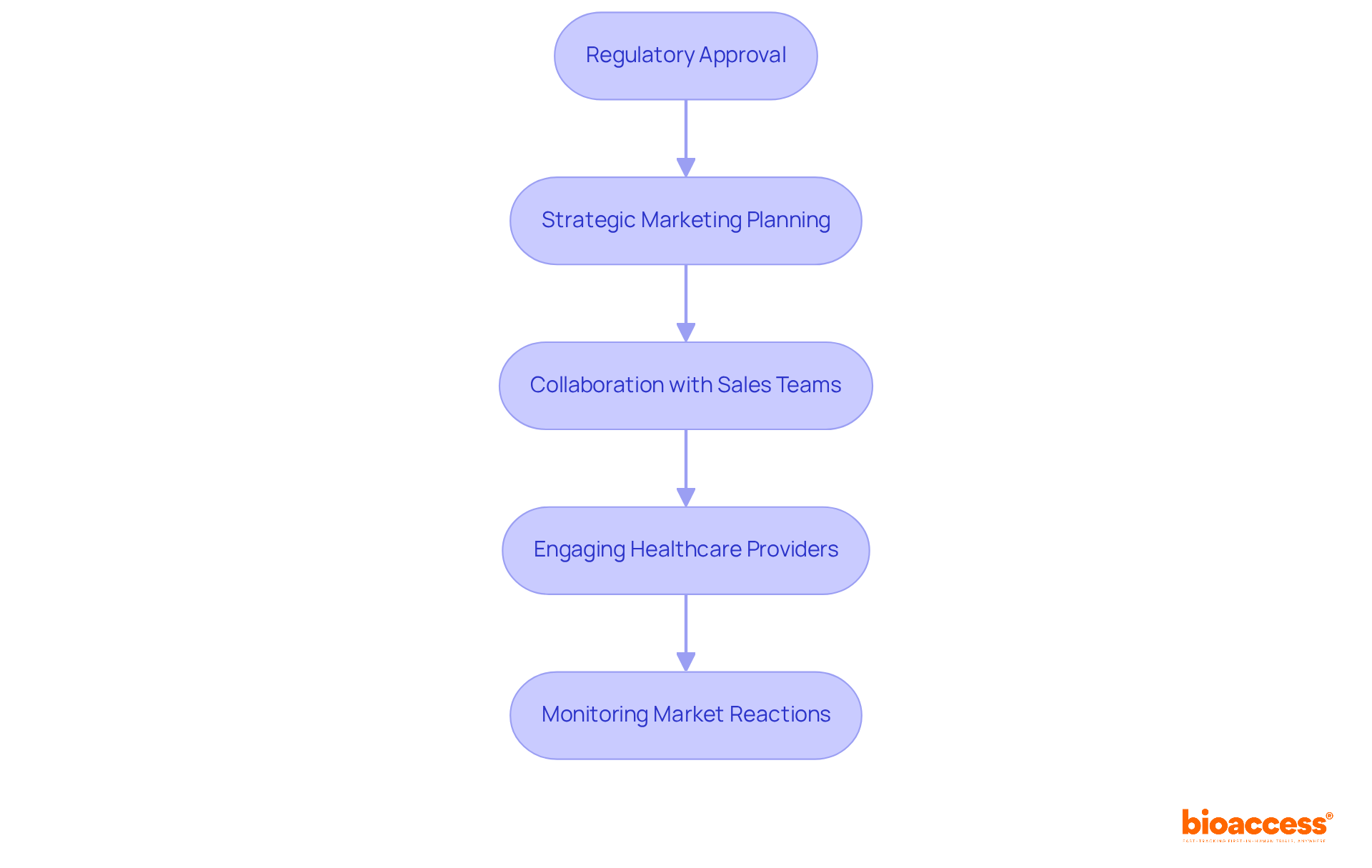
The launch phase is critical in clinical research, encompassing not just the acquisition of regulatory approval but also the need for meticulous strategic marketing and distribution planning. Successful launches hinge on close collaboration with sales teams, ensuring the device is effectively positioned in the market. Engaging healthcare providers and stakeholders through demonstrations and educational initiatives significantly boosts visibility and adoption rates. For example, companies that prioritize evidence-based marketing strategies have cultivated enhanced trust and credibility among healthcare professionals, which is vital for long-term success.
Moreover, monitoring early market reactions is essential for making timely adjustments, ensuring that the offering meets the evolving demands of the market. With the medical equipment industry projected to reach a staggering global market size of $678.88 billion by 2025, effective market introduction planning is increasingly crucial. This strategic approach not only capitalizes on growth opportunities but also secures a competitive advantage.
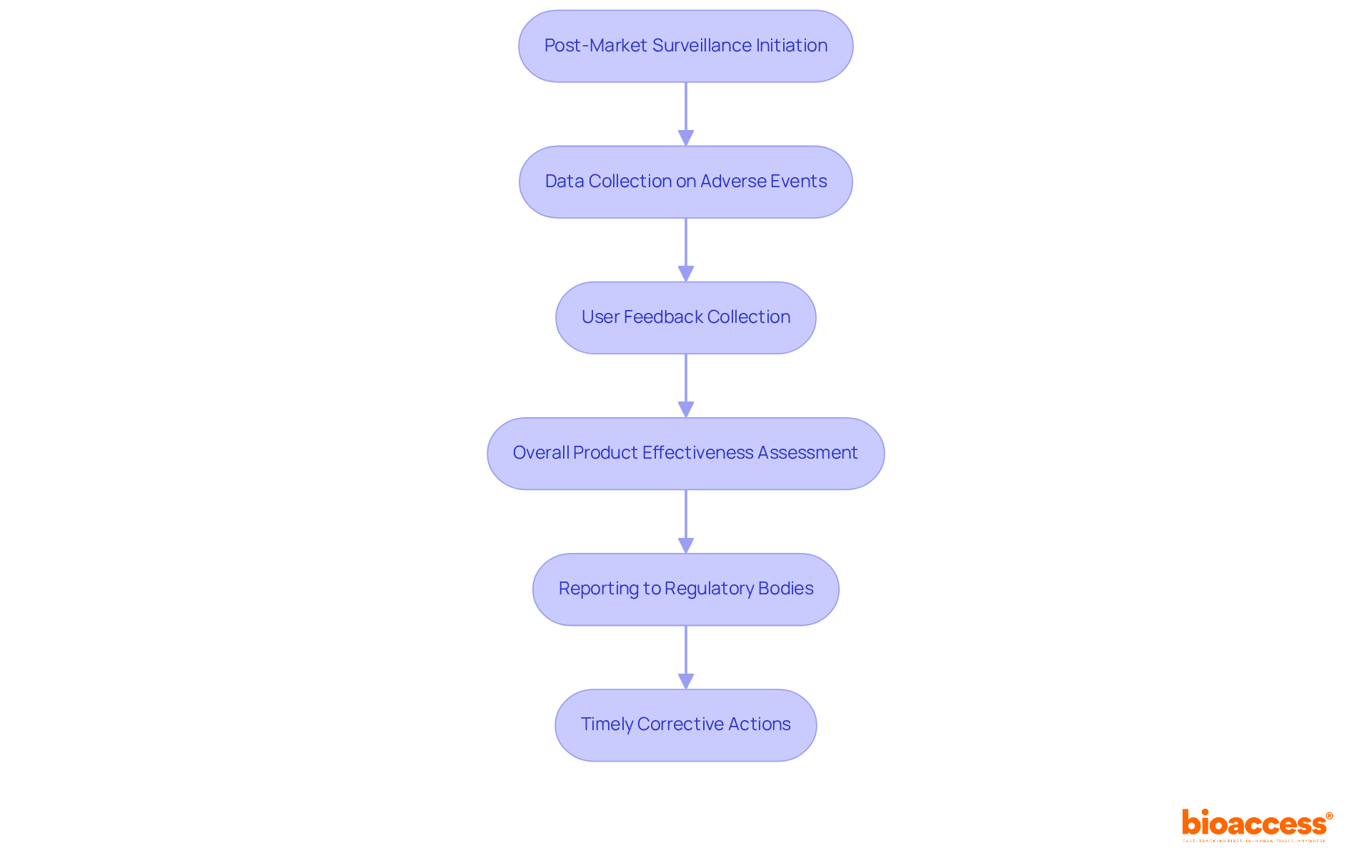
Post-market surveillance is crucial for evaluating the performance of medical equipment during the life cycle of medical devices in real-world settings after a product launch. This process involves systematically collecting data on adverse events, user feedback, and overall product effectiveness. Regulatory bodies require manufacturers to report this information regularly, highlighting the necessity for transparency and accountability in the industry. Did you know that around 40% of medical device adverse events are reported late? This statistic underscores the urgent need for proactive monitoring strategies.
At bioaccess®, we harness our expertise in managing Post-Market Clinical Follow-Up Studies (PMCF), Early-Feasibility Studies (EFS), and First-In-Human Studies (FIH) to establish a robust post-market surveillance plan. This comprehensive approach not only helps identify potential safety concerns early in the life cycle of medical devices but also encourages timely corrective actions, safeguarding individual health and ensuring compliance with standards. Companies that prioritize thorough monitoring strategies frequently report enhanced compliance outcomes, illustrating that effective performance monitoring transcends regulatory obligations - it's a vital aspect of patient safety and product quality.
Regulatory oversight plays a crucial role in clinical research, encompassing various agencies, such as the FDA in the United States. These organizations establish stringent guidelines for medical product development, ensuring that safety and efficacy are prioritized. Regulations cover the life cycle of medical device development, from the initial design phase to post-market monitoring, requiring manufacturers to demonstrate their products' reliability through rigorous testing and comprehensive documentation.
Staying informed about regulatory changes is essential for manufacturers. Open communication with regulatory bodies not only facilitates smoother approvals but also enhances the credibility of their offerings. As the Medtech landscape evolves, understanding these dynamics becomes increasingly important for addressing key challenges in clinical research. How prepared are you to navigate these complexities?

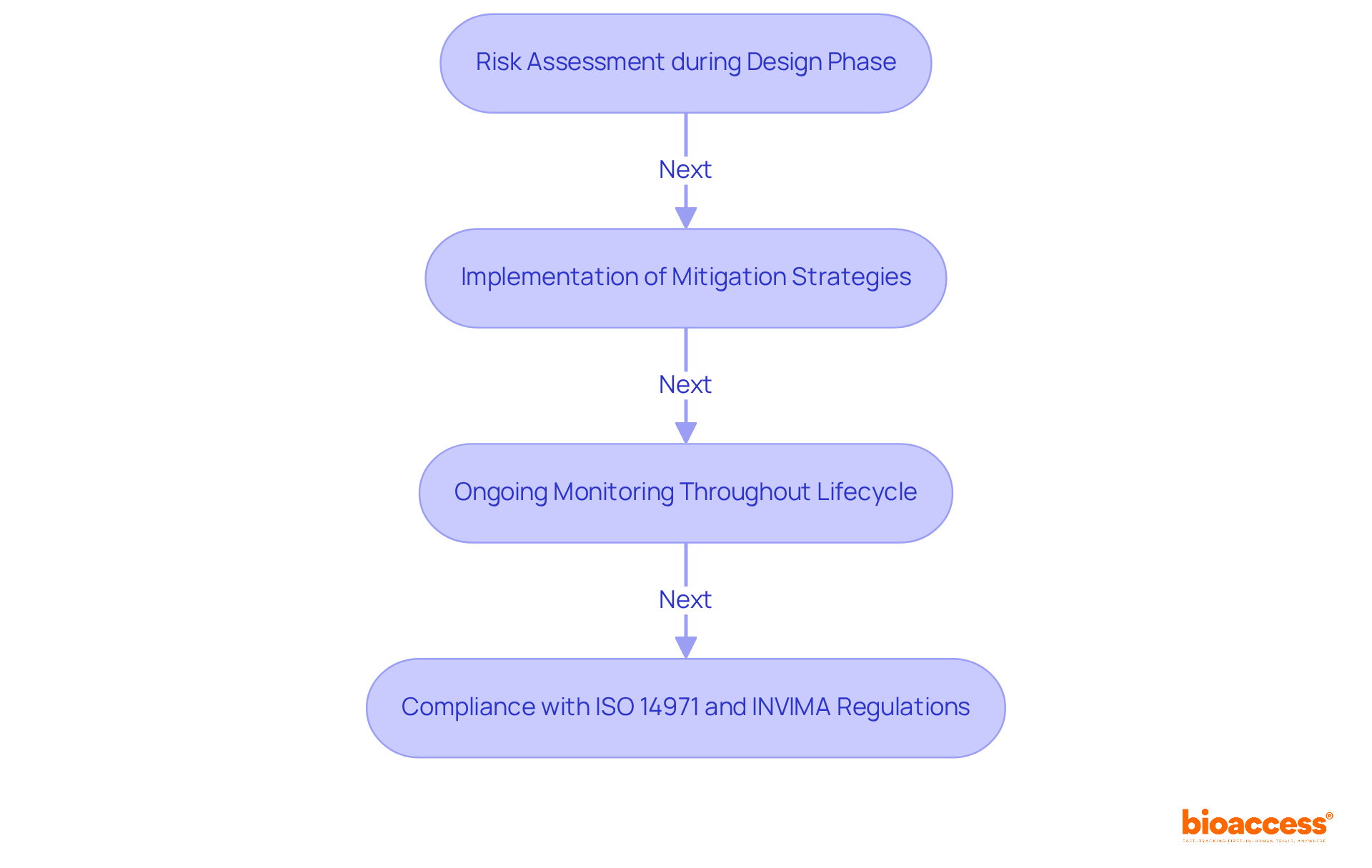
Risk management is crucial in the medical device sector, as it involves identifying potential hazards associated with equipment and implementing strategies to mitigate these risks. This process begins with thorough risk assessments during the design phase and continues with vigilant monitoring throughout the life cycle of the medical device. Manufacturers are required to comply with standards such as ISO 14971, which delineates the essential requirements for effective risk management in medical devices.
In Colombia, adherence to INVIMA regulations is not just important; it is mandatory. The Colombia National Food and Drug Surveillance Institute plays a pivotal role in overseeing the marketing and manufacturing of health products, ensuring that they meet stringent health standards. By prioritizing safety and proactively addressing risks, manufacturers can significantly enhance the reliability and acceptance of their products. This approach aligns with the best practices recommended by INVIMA, which is recognized as a Level 4 health authority by PAHO/WHO.
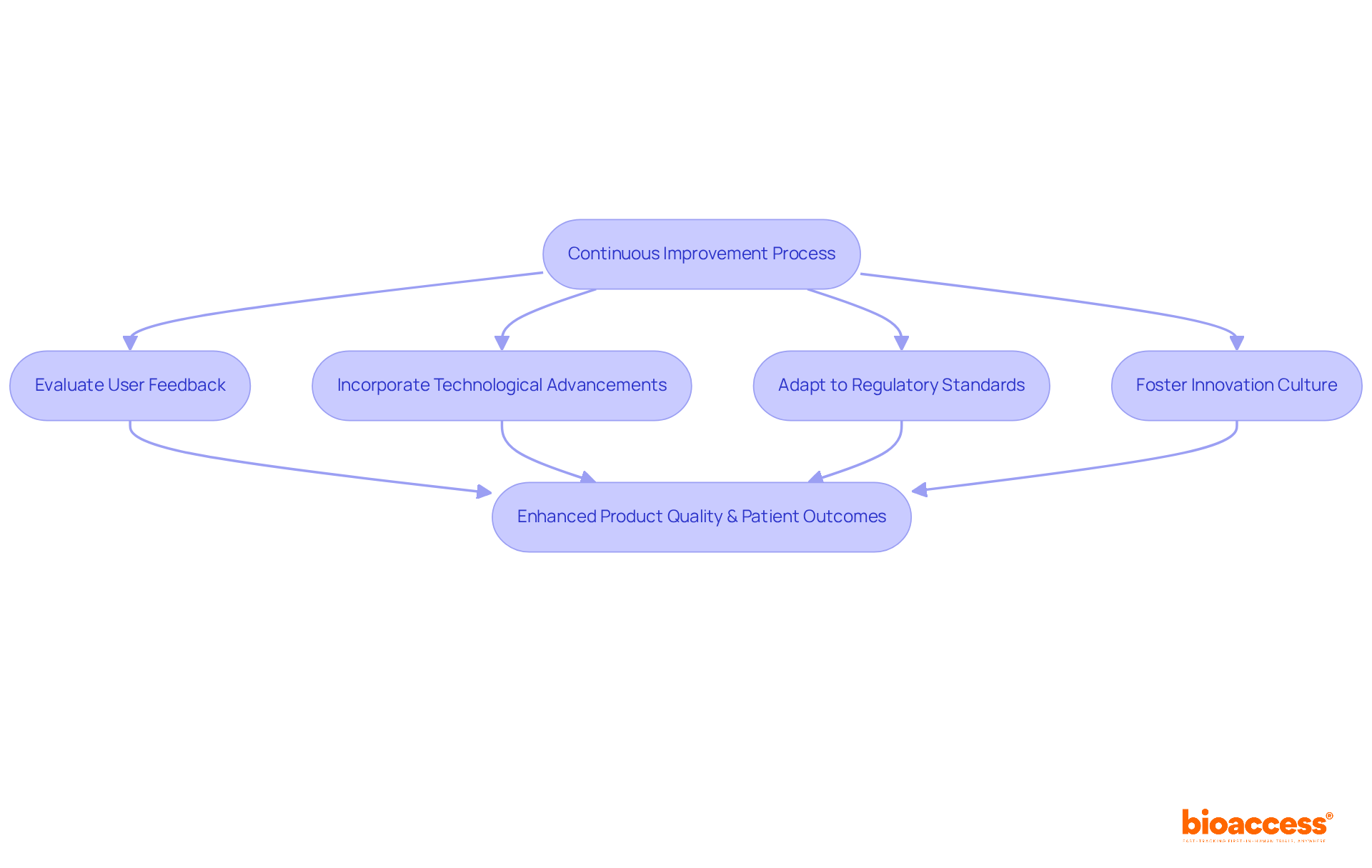
Ongoing enhancement is vital for the success of medical tools, compelling manufacturers to continuously evaluate and refine their offerings based on user feedback, technological advancements, and evolving regulatory standards.
Establishing a culture of innovation is essential; teams must be motivated to actively pursue improvements in design, functionality, and user experience.
By implementing structured feedback loops and performance metrics, organizations can identify opportunities for enhancement, ensuring that devices adapt to the dynamic needs of healthcare providers and patients alike.
This iterative process not only elevates product quality but also fosters innovation, ultimately leading to improved patient outcomes and heightened market competitiveness.
The life cycle of medical devices represents a complex journey, encompassing critical phases from initial conceptualization to post-market surveillance. For stakeholders in the Medtech industry, understanding these stages is not just beneficial; it’s essential. These phases serve as a roadmap, guiding the navigation through the intricate landscape of product development and regulatory compliance.
Key phases such as:
underscore the importance of a structured approach. This ensures that medical devices not only comply with regulatory standards but also effectively meet the needs of healthcare providers and patients. Collaborating with organizations like bioaccess® can significantly enhance this process, facilitating faster approvals and improving patient outcomes through efficient clinical trials.
Ultimately, embracing these phases and the insights they provide is crucial for driving innovation and maintaining competitiveness in the dynamic field of medical technology. Stakeholders must prioritize thorough research, engage proactively with regulatory bodies, and commit to continuous improvement. This approach is vital for the successful development and deployment of medical devices that enhance patient safety and care.
What is bioaccess® and how does it contribute to clinical research for medical devices?
bioaccess® specializes in expediting clinical research for medical devices by leveraging the regulatory advantages of Latin America, particularly Colombia, which offers significant cost savings and faster ethical approvals.
How much cost savings can be achieved by conducting clinical research in Colombia compared to North America and Western Europe?
Conducting clinical research in Colombia can lead to cost savings of over 30% compared to North America and Western Europe.
What is the timeframe for the IRB/EC and MoH review process in Colombia?
The total review process in Colombia takes only 90-120 days, with ethical approvals occurring within 4-6 weeks.
What advantages does Colombia's healthcare system provide for clinical research?
Colombia's healthcare system provides access to a diverse population of over 50 million, with 95% covered by universal healthcare, which accelerates enrollment by 50% compared to traditional markets.
What are the key phases in the development of medical devices as outlined in the article?
The key phases include Phase 1: Conceptualization and Market Need Identification, and Phase 2: Strategic Planning and Regulatory Considerations.
What is the focus of Phase 1 in the development process?
Phase 1 focuses on identifying a specific medical need and creating a solution that addresses this gap, supported by thorough market research and insights from healthcare professionals.
What strategies are recommended for effective market research in Phase 1?
Recommended strategies include quantitative surveys and qualitative interviews to gather diverse perspectives on existing solutions and their limitations.
What is the importance of strategic planning in Phase 2?
Strategic planning in Phase 2 involves creating a comprehensive plan that outlines the development timeline, budget, and compliance pathway, which is crucial for successful product development.
Why is communication with governing entities important during the regulatory process?
Clear communication with governing entities helps facilitate smoother approvals, identify potential hurdles early, and minimizes delays caused by compliance issues.
How can developers enhance their communication with oversight authorities?
Developers can enhance communication by engaging with local and national organizations, arranging regular meetings with oversight entities, and fostering a culture of adherence within their organizations.