


The key responsibilities of a sponsor of clinical trials encompass:
These responsibilities are crucial for the integrity and success of clinical research. Adherence to regulations is paramount, as it safeguards the study's credibility and protects participants. Effective investigator selection not only enhances outcomes but also fosters a collaborative environment essential for clinical trials. Proactive risk management is vital to safeguard participants, while the necessity of ethical practices builds trust among stakeholders. Collectively, these elements underscore the significance of the sponsor's role in navigating the complexities of clinical research.
The landscape of clinical trials is rapidly evolving, presenting sponsors with increasing pressure to navigate complex regulatory environments while ensuring participant safety and data integrity.
With the stakes higher than ever, grasping the key responsibilities of a clinical trial sponsor is paramount for success.
This article delves into the essential duties that sponsors must uphold, exploring how strategic support from expert organizations like bioaccess® can significantly enhance trial efficiency and compliance.
As the industry prepares for new regulatory changes in 2025, sponsors must consider how to effectively balance the demands of innovation with the rigorous standards required for ethical research.
bioaccess® excels in accelerating trials by leveraging its deep expertise in navigating the regulatory frameworks of Latin America, the Balkans, and Australia. With ethical approvals secured in a remarkable 4-6 weeks and enrollment processes that are 50% faster than traditional markets, bioaccess® provides a significant advantage to the sponsor of clinical trials in swiftly bringing their innovations to market. This efficiency is particularly advantageous for Medtech, Biopharma, and Radiopharma companies aiming to streamline their clinical research timelines.
The comprehensive services offered by bioaccess® encompass:
This guarantees that backers receive comprehensive reporting on study status and adverse events, fostering trust and transparency.
Significantly, the Latin America research market is anticipated to reach USD 2,654.0 million by 2030, underscoring the region's increasing significance with a compound annual growth rate (CAGR) of 6.7% from 2024 to 2030. As Phase I studies emerge as the fastest-growing segment, bioaccess®'s capabilities align perfectly with the evolving demands of sponsors of clinical trials seeking rapid and effective solutions. Collaboration with bioaccess® not only addresses current challenges but also positions the sponsor of clinical trials for success in a competitive landscape.
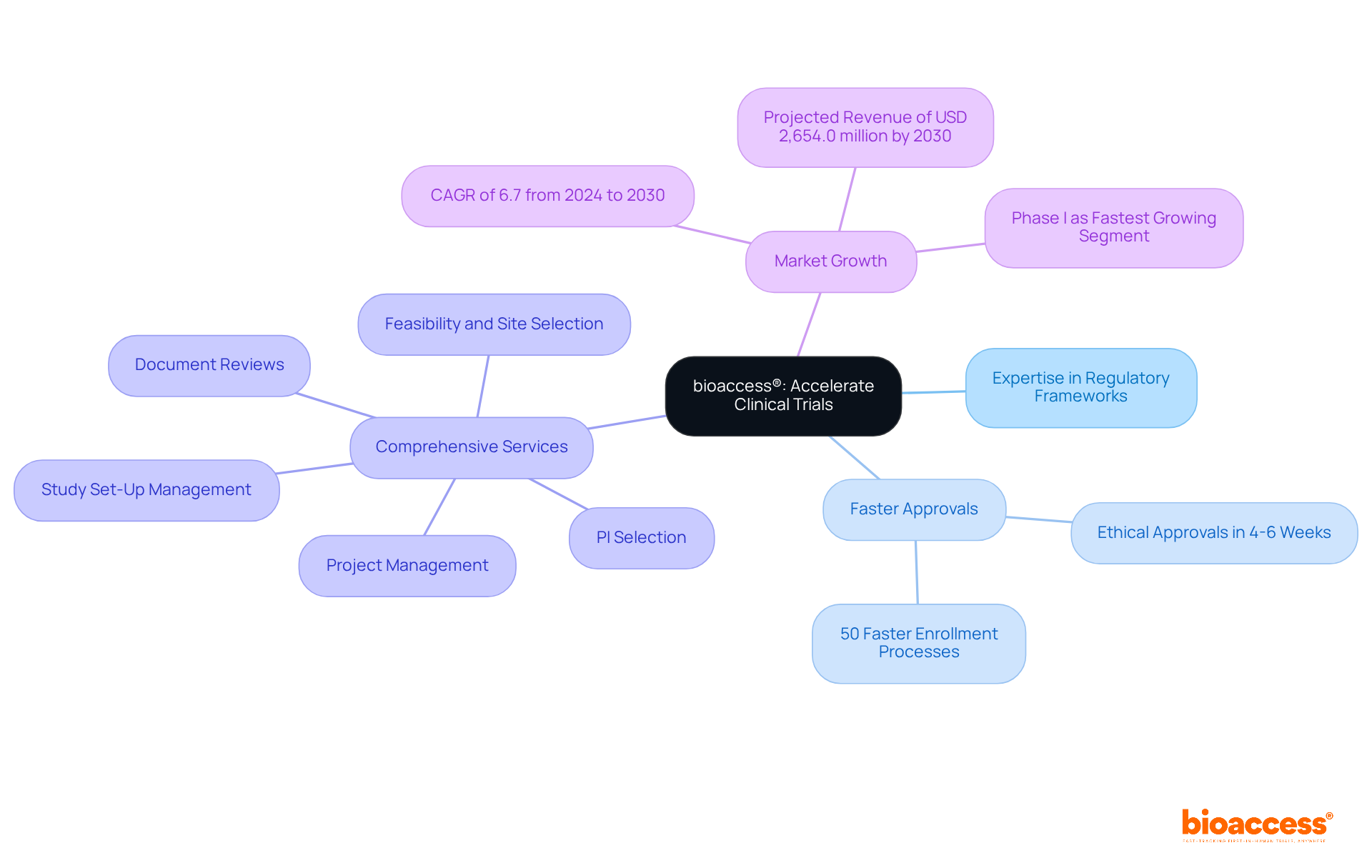
The sponsor of clinical trials plays a vital role in ensuring that clinical studies comply with all relevant regulations, particularly those established by the FDA. This responsibility encompasses acquiring essential approvals, which may necessitate approximately 4 to 6 weeks for ethical assessments, as well as understanding the specific criteria for each phase of the study.
It is crucial to maintain accurate documentation and align all trial activities with Good Clinical Practice (GCP) guidelines. Non-compliance can lead to significant delays, with the average cost of developing a new drug reaching around $2.8 billion. Furthermore, it can result in legal repercussions, including the withdrawal of drugs post-approval, which occurs in about 4% of cases due to safety concerns. Notably, 20% of medications receive new black box warnings after entering the market, underscoring the importance of continual adherence and monitoring.
As the FDA prepares to align guidance on single Institutional Review Board (IRB) reviews for multicenter studies in early 2025—an initiative anticipated to minimize duplication and standardize requirements—sponsors of clinical trials must stay informed about these updates to streamline their compliance processes.
Collaborating with regulatory specialists, such as those at bioaccess®, can provide valuable insights into navigating the complexities of FDA approval procedures and ensure that studies are organized effectively, from feasibility research to site selection and regulatory assessments.
As noted by Infonetica, 'Your commitment to compliance directly impacts the lives of countless individuals relying on medical breakthroughs,' highlighting the critical nature of these responsibilities.

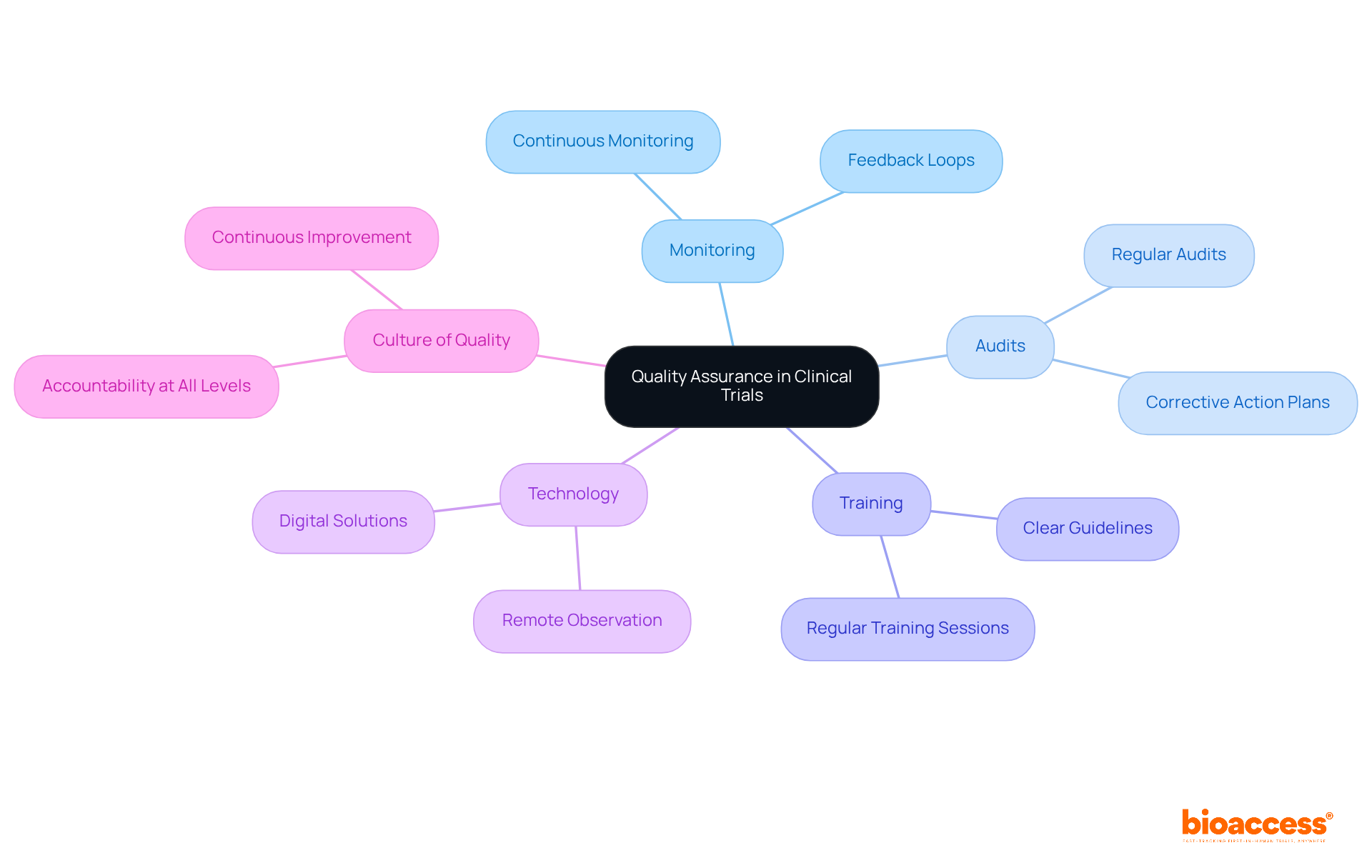
Establishing a robust quality assurance (QA) framework is crucial for backers to ensure that clinical studies adhere to established protocols and regulatory standards. This necessitates regular monitoring of experimental processes, conducting thorough audits, and formulating corrective action plans for any identified issues. By prioritizing QA, the sponsor of the clinical trial can significantly bolster data integrity, protect participant safety, and ultimately enhance the reliability of trial results.
Effective QA frameworks in clinical research typically incorporate continuous monitoring and feedback loops, facilitating real-time adjustments to protocols. Organizations that foster a culture of quality, where every team member is accountable for maintaining standards, often experience improved adherence and outcomes. This approach aligns with the philosophy that quality is not merely an act but a habit, underscoring the necessity for an ongoing commitment to excellence.
To implement QA effectively, the sponsor of the clinical trial should establish clear guidelines and training programs that empower staff to proactively recognize and address potential quality issues. Regular training sessions can underscore the significance of adherence and the role of each team member in maintaining study integrity. Furthermore, leveraging technology for remote observation has become increasingly essential, particularly in light of the changes initiated by the COVID-19 pandemic, which has accelerated the adoption of digital solutions in medical studies.
As we approach 2025, updates in clinical study integrity and compliance highlight the need for the sponsor of clinical trials to stay informed about evolving regulations and best practices. This includes adapting to new quality indicators and ensuring that all testing activities are meticulously documented to support audits and inspections.
QA experts emphasize that maintaining high standards of testing integrity is a collective responsibility. As one expert noted, 'Quality is the result of high intention, sincere effort, intelligent direction, and skillful execution.' By fostering an environment that prioritizes quality at every level, supporters can not only meet regulatory obligations but also enhance the overall success of their research studies.
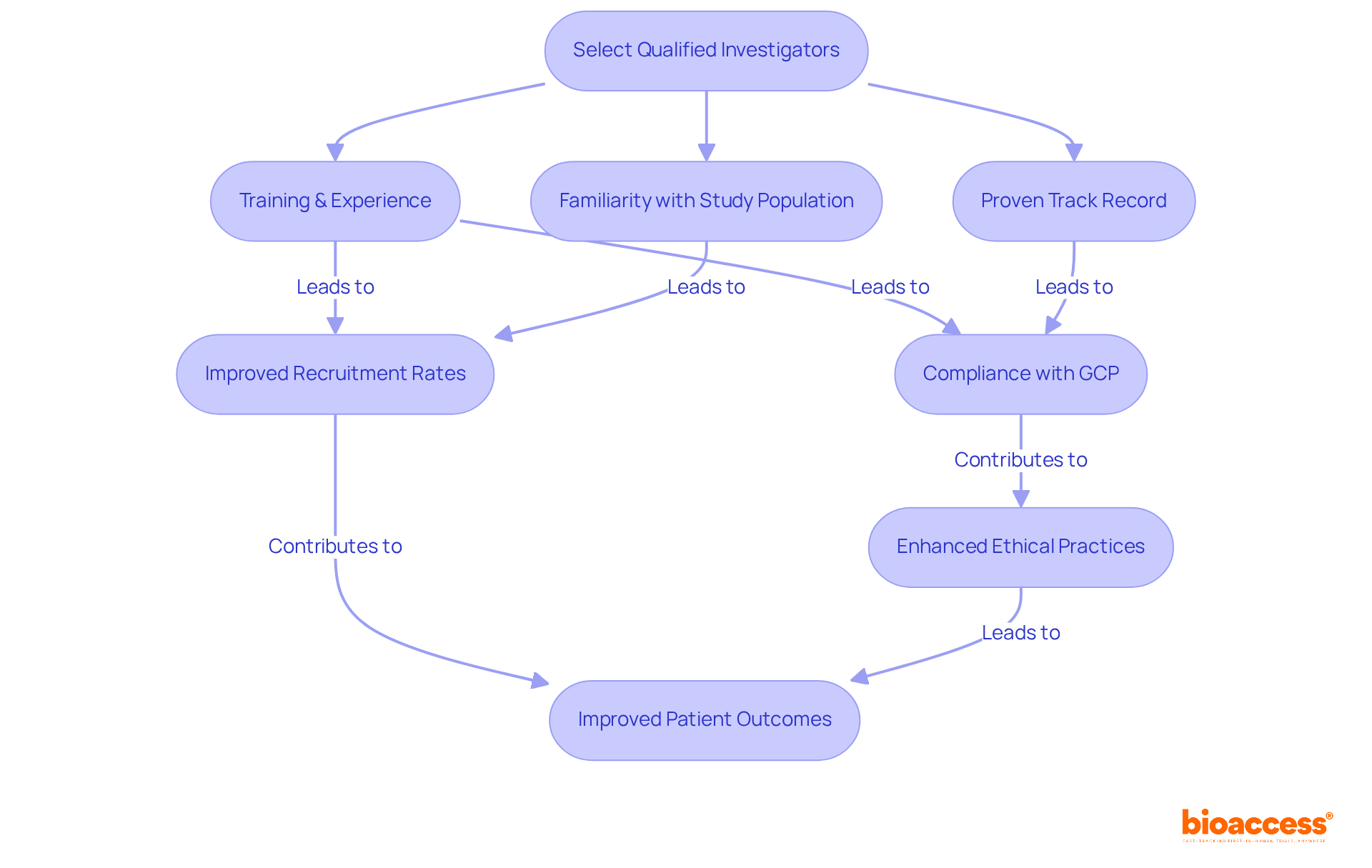
Choosing skilled researchers is a fundamental duty of the sponsor of clinical trial, as their expertise directly impacts the results of the study. Investigators must not only have the requisite training and experience but also a proven track record in conducting research pertinent to the study population. Research indicates that the success rate of medical studies can be significantly influenced by the investigator's prior experience, with successful researchers often demonstrating a history of effective patient recruitment and adherence to regulatory standards.
In 2025, the importance of selecting the right investigator has become increasingly critical as the research landscape evolves. A well-chosen investigator can enhance ethical practices and efficiency in legal proceedings, ultimately leading to improved patient outcomes. As Julia Garcia-Diaz, a prominent figure in medical research, notes, "Investigators are responsible for producing high-quality, meaningful scientific research, but they are also responsible for maintaining public trust."
Furthermore, the selection process should take into account the investigator's familiarity with the study population, as this can streamline participant recruitment and retention. For instance, studies that engage researchers with established community connections often report heightened enrollment rates, which is essential given that approximately 80% of medical studies fail to meet their initial enrollment objectives, resulting in significant financial repercussions.
Effective investigator selection not only ensures compliance with Good Clinical Practice (GCP) guidelines but also fosters collaboration among stakeholders, which enhances the overall quality of research, as emphasized by the sponsor of clinical trial. By prioritizing skilled and well-connected researchers, the sponsor of clinical trial can significantly enhance the likelihood of success, ultimately advancing the development of new therapies and improving patient care.
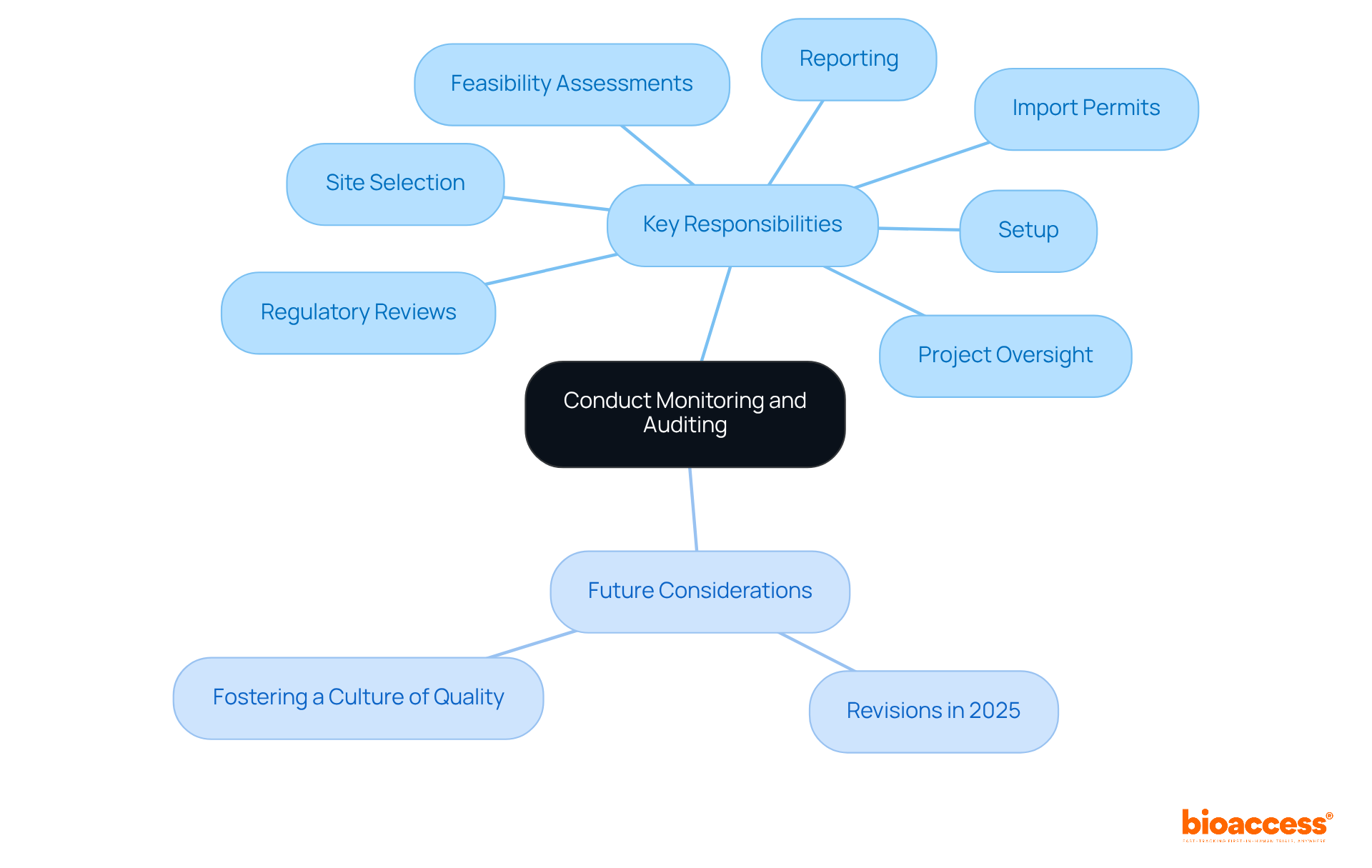
The sponsor of clinical trial plays a crucial role in ensuring the integrity of clinical studies through regular monitoring and auditing, which upholds compliance with protocols and regulatory standards. This responsibility encompasses a comprehensive review of data collection processes, verification of participant safety measures, and assessment of overall conduct. Effective monitoring is essential; it aids in recognizing potential issues early and allows stakeholders to implement corrective actions swiftly. For instance, bioaccess provides extensive study management services, including:
Looking ahead, revisions to research standards in 2025 will further underscore the significance of these practices, reinforcing the necessity for backers to adapt and enhance their oversight strategies. Industry experts emphasize that fostering a culture of quality, which prioritizes continuous improvement over mere compliance, is vital for achieving reliable outcomes. By employing innovative monitoring methods and promoting collaboration among stakeholders, a sponsor of clinical trial like bioaccess can significantly enhance the integrity of research studies, ultimately leading to more successful and ethical research outcomes.
Active involvement with regulatory bodies is crucial for backers navigating the intricate landscape of clinical study regulations. This encompasses not only the submission of essential documentation but also the prompt response to inquiries and active engagement in discussions regarding the project's progress and regulatory challenges. By cultivating robust connections with regulators, the sponsor of the clinical trial can streamline the approval process and proactively address any issues that may arise during the study.
Bioaccess® offers specialized services that bolster this engagement, including:
These services are strategically designed to enhance communication strategies, such as regular updates and transparent dialogue, significantly improving compliance navigation. As regulatory landscapes evolve, particularly with anticipated updates in 2025, maintaining open lines of communication becomes increasingly vital. The impact of these regulatory connections is profound, often resulting in shorter approval durations and a more efficient testing process, ultimately benefiting both the sponsor of clinical trials and the participants.

To ensure participant safety and uphold the integrity of study data, the sponsor of the clinical trial must implement robust risk management strategies. This essential process begins with identifying potential risks associated with the experiment, followed by a thorough assessment of their likelihood and impact. Developing comprehensive mitigation plans is crucial for effectively addressing these risks. Proactive risk management not only enhances participant safety but also ensures compliance with regulatory standards and maintains the credibility of study outcomes. Notably, 70% of research sites have recognized a lack of time for investigators as a significant risk, underscoring the necessity for effective resource distribution. Furthermore, the implementation of systematic risk management tools can help anticipate difficulties, with 88.5% of sites reporting improved patient safety as a direct benefit. By fostering a culture of risk awareness and employing effective strategies, a sponsor of clinical trial can significantly enhance the overall safety and success of research studies.
Guaranteeing precise data gathering and reporting is a fundamental responsibility of the sponsor of clinical trial throughout the clinical study process. This responsibility encompasses:
By prioritizing data integrity, the sponsor of clinical trial not only enhances the reliability of study outcomes but also supports informed decision-making in the development of new therapies. As industry specialists assert, precise reporting is vital for sustaining trust in research, directly affecting the credibility of findings and the subsequent endorsement of new therapies.
For instance, bioaccess provides extensive research management services, including:
These services are essential for ensuring that data collection processes are robust and dependable. Implementing strong data validation techniques and conducting regular audits can significantly mitigate risks associated with data inaccuracies. Ultimately, the influence of data integrity on research outcomes cannot be overstated; it serves as the foundation for effective regulatory submissions and the successful commercialization of innovative therapies.
The sponsor of clinical trial plays a crucial role in the success of medical studies by providing essential resources, including funding, personnel, and access to necessary facilities and equipment. The general success rate of medical studies is only 7.9%, emphasizing the significance of sufficient assistance in improving study results. By ensuring that researchers and research locations are well-equipped, backers can facilitate smoother operations and significantly enhance the chances of successful outcomes.
For instance, a study uncovered that nearly 80% of research studies do not achieve their original enrollment targets, resulting in significant revenue losses—about $8 million daily for pharmaceutical development firms. This underscores the necessity for the sponsor of clinical trials to implement effective patient recruitment strategies and allocate sufficient resources to meet enrollment targets.
A significant instance of successful teamwork is the alliance between bioaccess™ and Caribbean Health Group, which seeks to establish Barranquilla as a premier location for medical studies in Latin America. Supported by Colombia's Minister of Health, this initiative not only enhances the clinical research landscape but also contributes to local economic growth through job creation and improved healthcare services.
Clinical study managers emphasize that proper documentation and proactive risk management are crucial for effective sponsor oversight. As one manager noted, 'If it wasn’t documented, it wasn’t done,' stressing the need for meticulous record-keeping to ensure compliance and accountability.
Moreover, successful examples of resource allocation can be seen in organizations like Cedar Health Research, which improved patient engagement by targeting diverse audiences with culturally relevant outreach while utilizing integrated eClinical platforms. Such strategies not only improve recruitment but also cultivate trust and guarantee that studies meet the genuine needs of the populations they intend to help.
In 2025, as the clinical research environment changes, sponsors of clinical trials must prioritize offering essential resources to facilitate study execution efficiently. This involves investing in advanced technologies, maintaining clear communication with study locations, and ensuring diversity in participant recruitment to reflect the populations most likely to benefit from the research. By doing so, a sponsor of clinical trials can significantly enhance success rates of studies and contribute to more equitable health outcomes.

The sponsor of clinical trial plays a crucial role in maintaining the highest ethical standards throughout the research process, which is vital for safeguarding the rights and welfare of participants. This obligation includes acquiring informed consent, protecting participant confidentiality, and carrying out studies in a way that reduces risks. A systematic review revealed that understanding of informed consent components varied significantly, with only 75.8% of participants aware of their right to withdraw at any time, while awareness of randomization was at just 52.1%. These statistics highlight the ongoing challenges in ensuring comprehensive participant understanding.
By prioritizing ethical considerations, a sponsor of clinical trials can foster trust with participants and stakeholders, which is crucial for the integrity and success of medical research. The dedication to openness and responsibility is evident in recent efforts focused on integrating research studies within national health frameworks and promoting diverse participant involvement. For example, bioaccess provides extensive study management services, including:
These services ensure that study designs are guided by systematic reviews to address evidence gaps, thereby enhancing the relevance and impact of research studies.
In 2025, updates to ethical practices emphasize the importance of protecting participant rights, with a focus on clear communication regarding data sharing and informed consent processes. This commitment not only aligns with the ethical imperative of transparency but also builds trust among trial participants and the public, ultimately contributing to more effective and responsible clinical research.

The responsibilities of a sponsor in clinical trials are multifaceted and critical to the success of research endeavors. By ensuring regulatory compliance, implementing quality assurance, selecting qualified investigators, and engaging with regulatory authorities, sponsors play a pivotal role in advancing medical research while safeguarding participant welfare.
Key points throughout the article highlight the importance of robust risk management strategies, accurate data collection, and the provision of essential resources. The significance of ethical standards and participant protection is underscored, emphasizing that a sponsor's commitment to compliance and quality directly influences the integrity of clinical studies and the safety of participants. Furthermore, collaboration with specialized entities like bioaccess® can enhance the efficiency and effectiveness of the clinical trial process.
Ultimately, the role of a clinical trial sponsor extends beyond mere oversight; it encompasses a commitment to excellence that fosters trust and transparency in the research community. As the landscape of clinical research evolves, sponsors must prioritize their responsibilities to ensure that medical innovations reach the market safely and effectively, ultimately benefiting patients and society as a whole. Engaging with these principles not only enhances research outcomes but also reinforces the ethical foundation upon which clinical trials are built.
What is bioaccess® and what services does it provide for clinical trials?
bioaccess® is a company that accelerates clinical trials by leveraging its expertise in navigating regulatory frameworks in Latin America, the Balkans, and Australia. Its services include feasibility and selection of research sites, principal investigator selection, reviews of study documents for compliance, management of study set-up and approvals, and continuous project management and oversight.
How quickly can bioaccess® secure ethical approvals for clinical trials?
bioaccess® can secure ethical approvals in a remarkable 4 to 6 weeks, which is significantly faster than traditional markets.
What advantages does bioaccess® offer to sponsors of clinical trials?
bioaccess® offers significant advantages by providing enrollment processes that are 50% faster than traditional markets, allowing sponsors to bring their innovations to market more swiftly. This efficiency is particularly beneficial for Medtech, Biopharma, and Radiopharma companies.
What is the projected growth of the Latin America research market?
The Latin America research market is anticipated to reach USD 2,654.0 million by 2030, with a compound annual growth rate (CAGR) of 6.7% from 2024 to 2030.
What are the key responsibilities of sponsors in ensuring regulatory compliance for clinical trials?
Sponsors are responsible for ensuring compliance with all relevant regulations, including acquiring necessary approvals, maintaining accurate documentation, and aligning trial activities with Good Clinical Practice (GCP) guidelines. Non-compliance can lead to significant delays and legal repercussions.
What are the potential consequences of non-compliance in clinical trials?
Non-compliance can result in significant delays in drug development, legal repercussions, and the potential withdrawal of drugs post-approval due to safety concerns. Approximately 20% of medications receive new black box warnings after entering the market.
How can sponsors enhance trial integrity and compliance through quality assurance (QA)?
Sponsors can enhance trial integrity by establishing a robust QA framework that includes regular monitoring, thorough audits, and corrective action plans. This approach helps bolster data integrity, protect participant safety, and improve trial results.
What role does technology play in quality assurance for clinical trials?
Technology facilitates remote observation and real-time adjustments to protocols, which has become essential, especially due to the changes initiated by the COVID-19 pandemic. It helps maintain high standards of testing integrity and supports audits and inspections.
What is the importance of training in implementing quality assurance?
Training is crucial for empowering staff to recognize and address potential quality issues proactively. Regular training sessions emphasize the significance of adherence to protocols and the role of each team member in maintaining study integrity.
Why is it important for sponsors to stay informed about evolving regulations and best practices?
Staying informed about evolving regulations and best practices is essential for sponsors to ensure compliance, adapt to new quality indicators, and maintain meticulous documentation of testing activities, which supports audits and inspections.