


This article outlines essential strategies for conducting clinical trials in medical devices, underscoring the critical role of regulatory efficiency, diverse patient pools, and streamlined ethical approvals. By leveraging these factors, particularly in regions such as Latin America and Australia, organizations can significantly reduce trial timelines and enhance the overall success of medical device research. Successful case studies and extensive experience in navigating complex regulatory environments further illustrate this point. As the Medtech landscape evolves, understanding these dynamics becomes increasingly relevant for clinical research stakeholders.
The landscape of clinical trials for medical devices is evolving rapidly, driven by the need for efficiency and innovation. Regulatory environments are shifting, and patient demographics are diversifying, creating abundant opportunities for Medtech companies to streamline their research processes. However, navigating these complexities raises critical questions: How can firms leverage strategic insights to enhance trial outcomes and expedite market entry? This article explores ten key strategies that not only address these challenges but also position organizations to thrive in the competitive Medtech arena.
bioaccess® excels in expediting research for medical instruments by leveraging the regulatory efficiency of Latin America, the diverse patient demographics of the Balkans, and the effective ethical approval procedures in Australia. This strategic approach enables the organization to secure ethical approvals in as little as 4-6 weeks, with enrollment rates that are 50% faster than traditional markets. Since its inception in 2010, bioaccess® has successfully completed over 159 regulatory submissions for more than 75 clinical trials in medical devices, demonstrating its expertise in navigating complex regulatory environments.
A notable illustration of this success is the case of Mitralign, a Boston-based company that encountered significant delays in Europe. By relocating its medical study to Colombia, Mitralign achieved ethical approval in a mere 18 days and enrolled 11 participants within eight weeks, ultimately accelerating its EU submission by 14 months. This exemplifies the tangible benefits of conducting research, particularly clinical trials in medical devices, in regions with more flexible regulatory procedures.
As of 2025, prevailing trends in medical device studies underscore the integration of advanced technologies such as AI and machine learning, which enhance data collection and analysis. bioaccess® remains at the forefront of these innovations, offering cutting-edge electronic data capture solutions that streamline the research process. With a dedicated team of nearly 40 experts and a network of over 150 active research sites worldwide, bioaccess® is distinctly positioned to assist Medtech innovators in achieving their research goals efficiently and effectively.

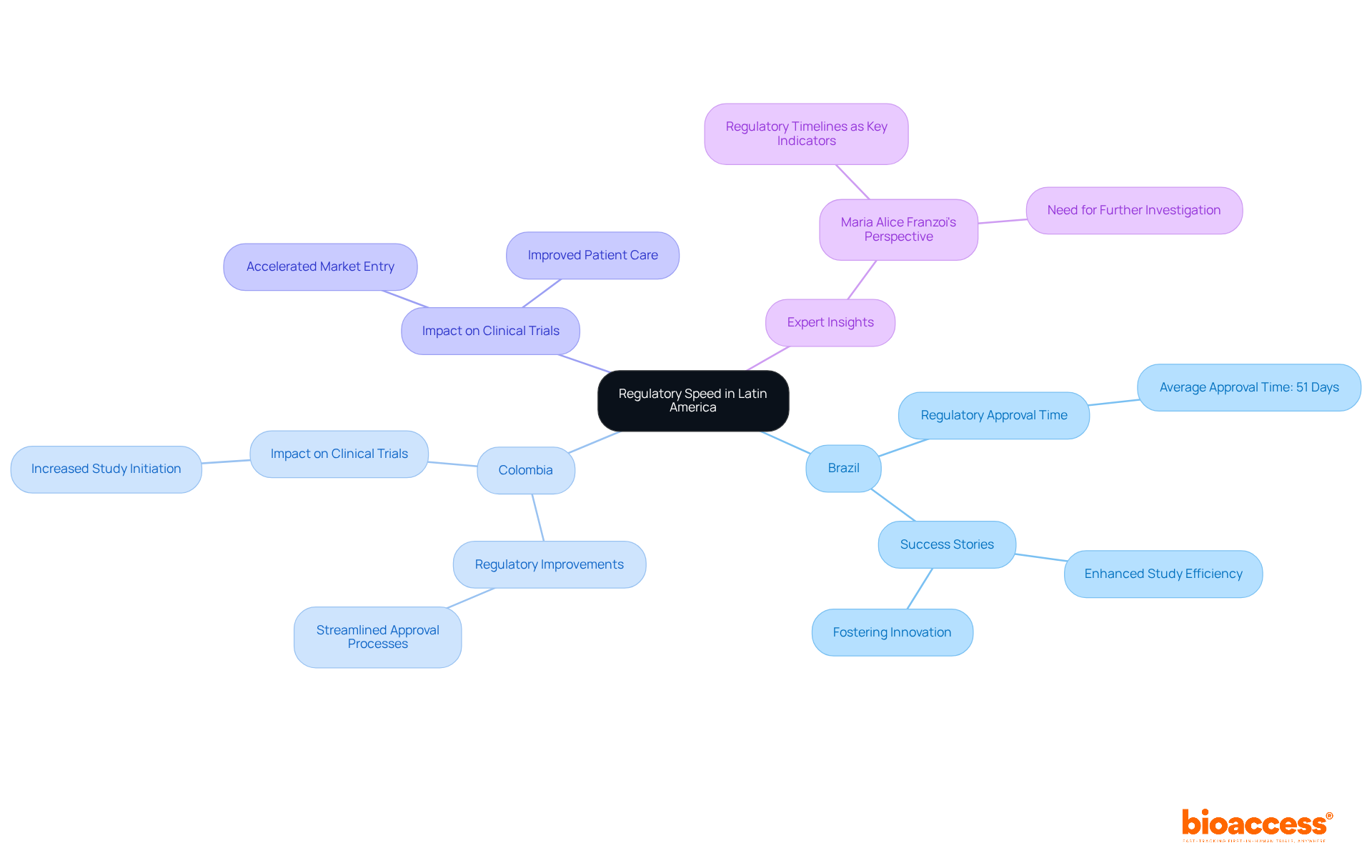
Latin America is recognized for its swift regulatory procedures, which play a pivotal role in accelerating research studies. In 2025, the region witnessed a substantial increase in the number of research studies initiated, underscoring its growing importance in the global Medtech landscape. Brazil and Colombia have made remarkable progress in refining their approval systems, leading to expedited study initiation.
For instance, Brazil's recent changes to its research approval process have reduced the average time to regulatory authority approval to approximately 51 days, a notable contrast to the prolonged timelines observed in many high-income countries. This regulatory agility is essential for Medtech companies striving to swiftly bring innovative devices to market, as it significantly shortens the timeline from concept to commercialization.
Success stories from Brazil and Colombia illustrate how these accelerated processes not only enhance the efficiency of studies but also foster an environment conducive to innovation, ultimately benefiting patient care and access to new technologies.
bioaccess® draws upon over 20 years of expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies to adeptly navigate these regulatory landscapes.
As noted by Maria Alice Franzoi from the Clinical Trials Support Unit, 'Regulatory timelines are regarded as one of the most crucial factors in conducting studies, and are viewed by pharmaceutical companies as key indicators of a nation’s appeal regarding the execution of a study.
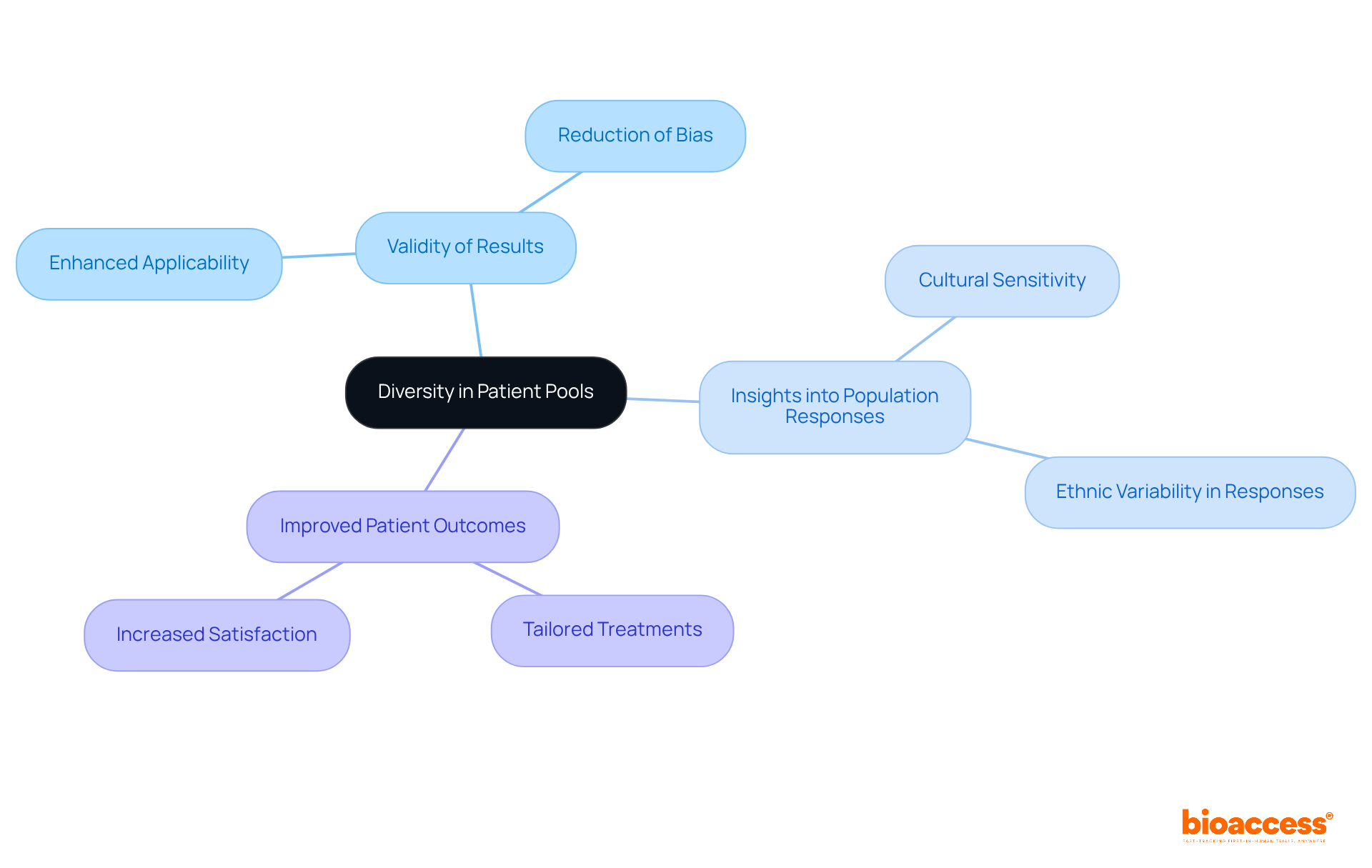
The Balkans present a diverse array of patient groups, which is vital for clinical research studies. By recruiting from these varied populations, researchers can ensure that their findings are applicable across different ethnic and cultural backgrounds. This diversity not only enhances the validity of the results of clinical trials in medical devices but also provides insights into how distinct populations respond to these devices. Ultimately, this understanding leads to improved patient outcomes, underscoring the critical role of diversity in the research landscape.
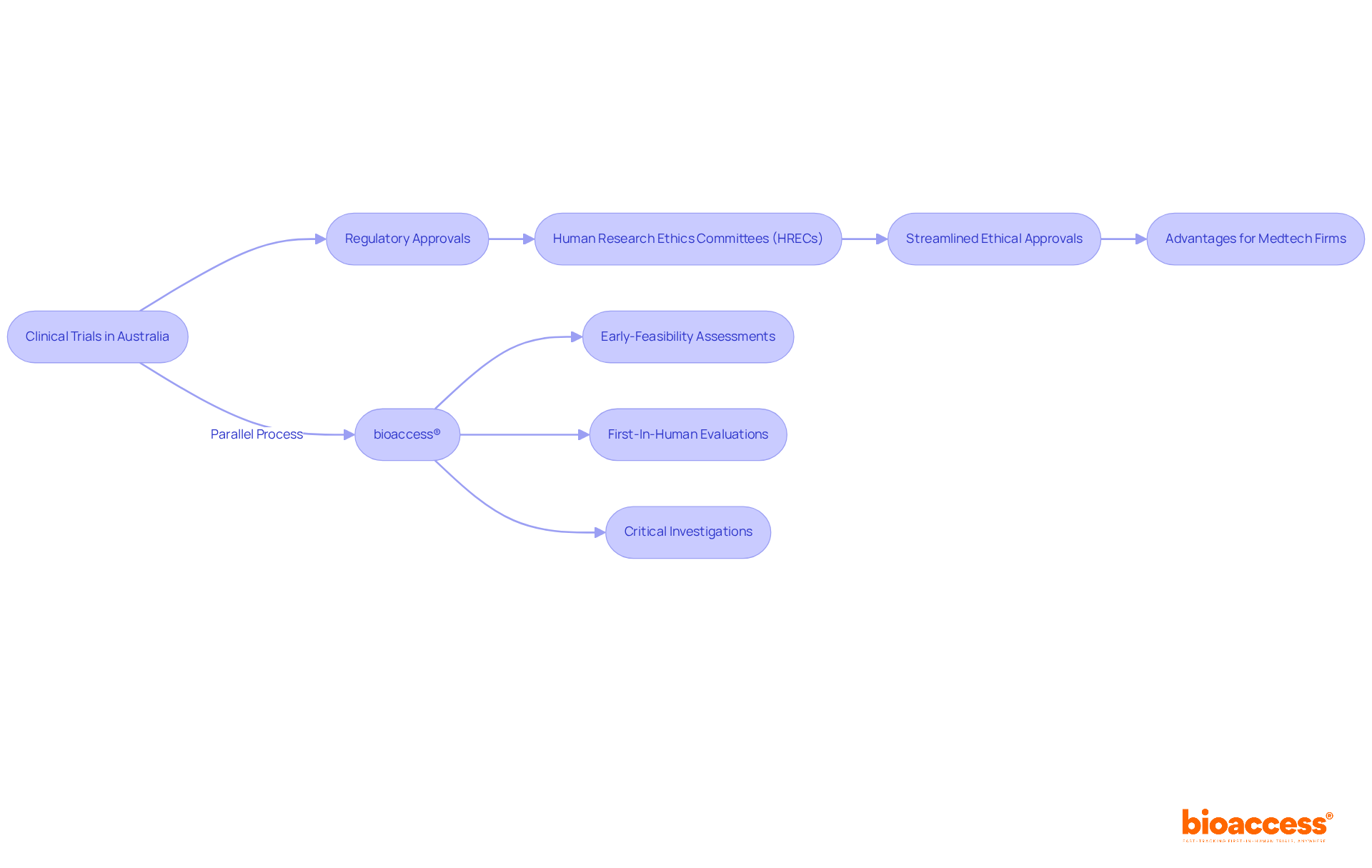
Streamlined Ethical Approvals: A Game Changer for Clinical Trials in Australia
Australia's ethical approval process is designed to be efficient, enabling clinical trials to commence swiftly once regulatory approvals are secured. The Human Research Ethics Committees (HRECs) play a crucial role in this process, ensuring that research meets ethical standards while minimizing delays. This streamlined method is particularly advantageous for Medtech firms seeking to conduct clinical trials in medical devices during early phases, as it significantly reduces the time to market.
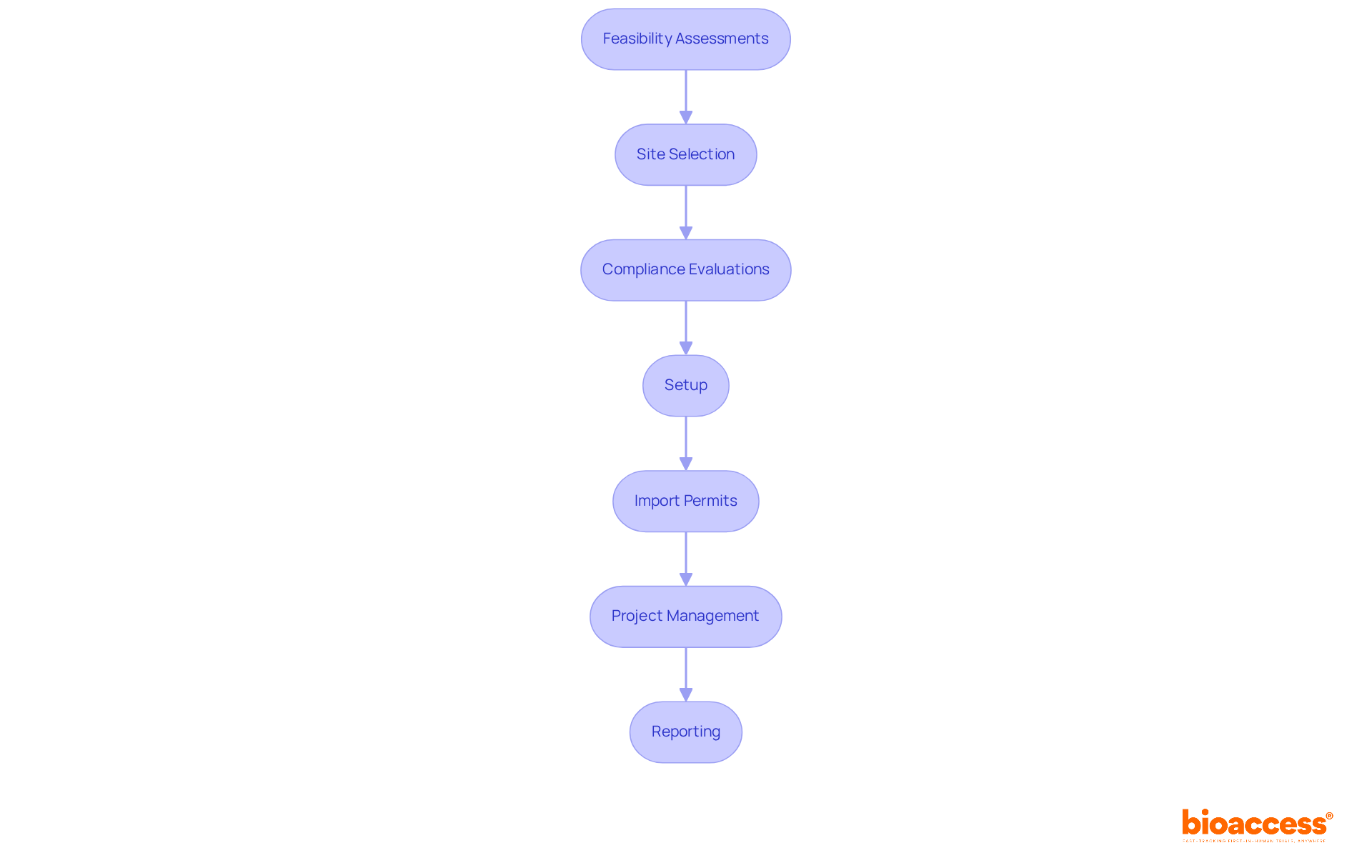
In contrast, bioaccess® offers specialized expertise in overseeing medical equipment clinical evaluations in Latin America, focusing on:
This dual perspective underscores the importance of understanding both local and international regulatory landscapes to optimize trial outcomes.
The collaboration between streamlined ethical approvals in Australia and bioaccess's insights into Latin America exemplifies the necessity for Medtech firms to navigate complex regulatory environments effectively. By leveraging these strengths, organizations can enhance their clinical research strategies and ultimately improve patient outcomes.
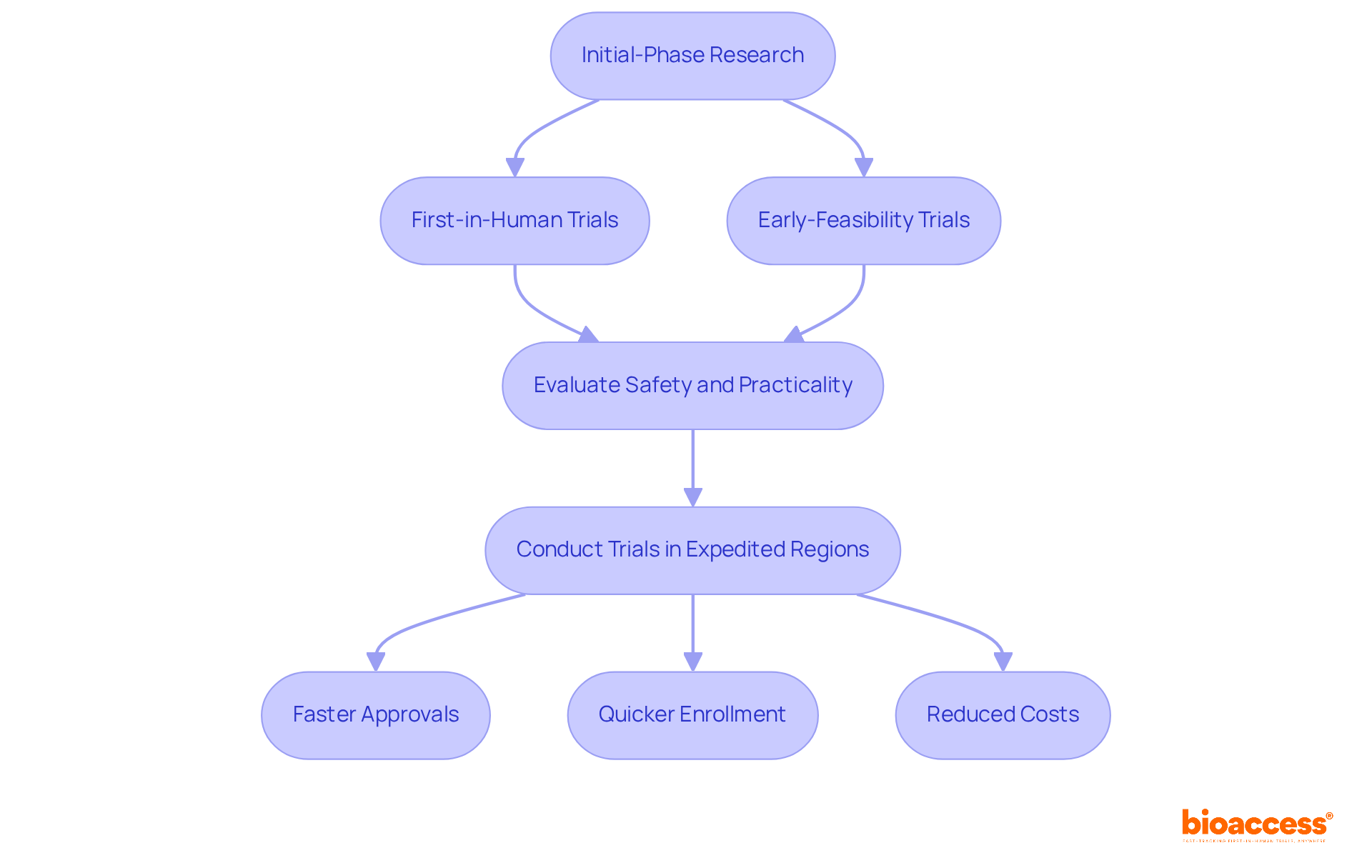
Initial-phase research, which includes first-in-human (FIH) and early-feasibility trials (EFS), is essential for evaluating new medical instruments in clinical trials in medical devices. These analyses not only help identify potential safety concerns but also assess the practicality of devices used in clinical trials in medical devices.
Conducting clinical trials in medical devices in regions with expedited regulatory processes, such as Latin America and Australia, enables Medtech companies to rapidly gather the necessary data to advance to larger trials.
Bioaccess® empowers innovators in Medtech, Biopharma, and Radiopharma to accelerate these initial investigations, achieving approvals up to 40% faster and enrollment 50% quicker, all while reducing costs by 30%.
For instance, Avantec Vascular has chosen Latin America for its first-in-human trial of an innovative vascular instrument, leveraging bioaccess®'s expertise in regulatory submissions and investigator selection. Similarly, ReGelTec's initial feasibility assessment of HYDRAFIL™ for treating chronic low back pain in Colombia showcases how bioaccess® facilitates swift medical advancements in the region.
Market access services are crucial for Medtech firms seeking to effectively commercialize their products in Latin America. bioaccess® provides comprehensive market access solutions that streamline the regulatory landscape, facilitating faster approvals and efficient market entry. Our extensive research management services encompass:
By leveraging bioaccess®'s expertise, including strategic reimbursement and health economics solutions, companies can adeptly navigate complex reimbursement pathways and engage effectively with healthcare providers. This strategic approach not only enhances the likelihood of successful market entry but also maximizes return on investment.
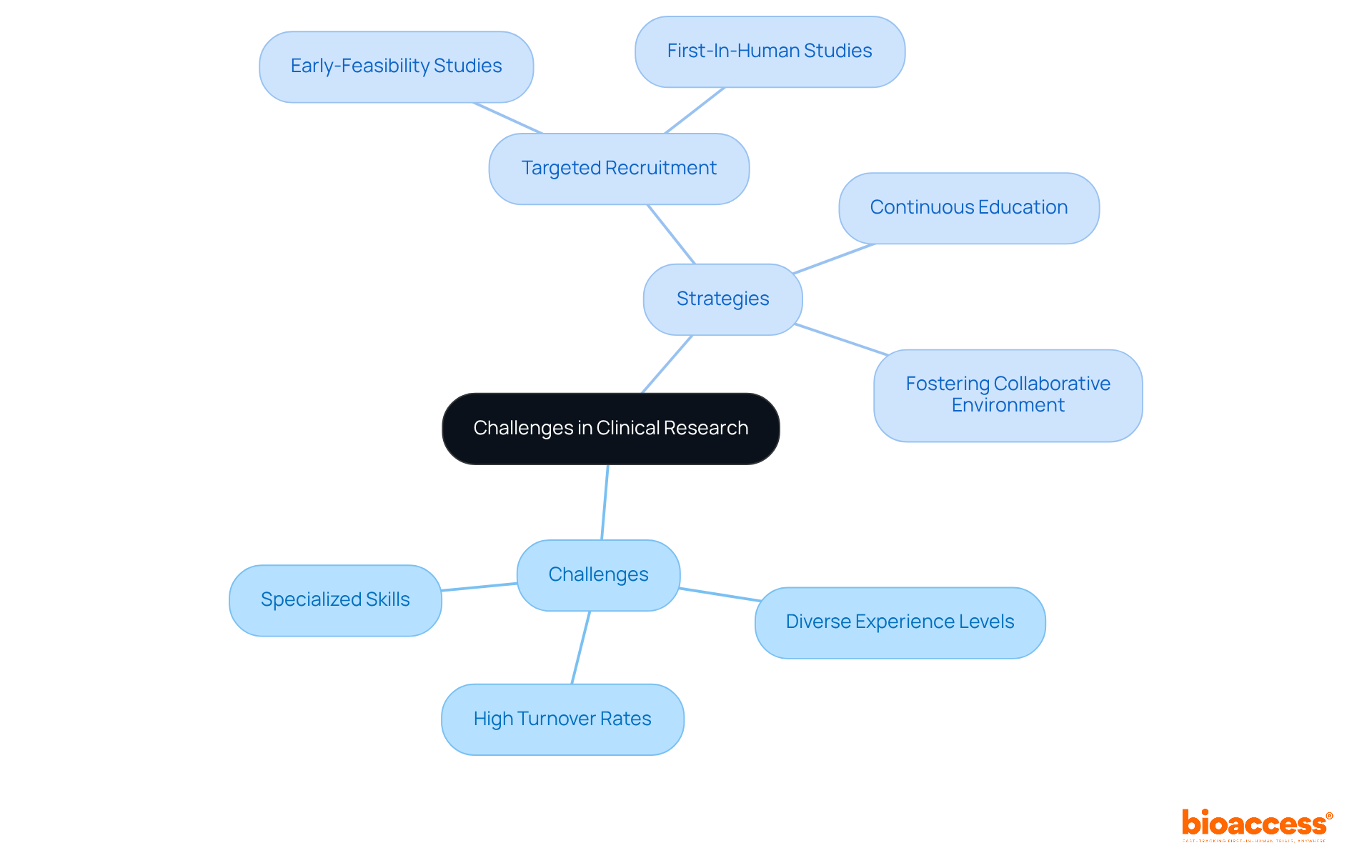
Recruiting and overseeing research groups presents significant challenges in clinical trials in medical devices, particularly concerning medical products. High turnover rates, diverse levels of experience, and the necessity for specialized skills can complicate team dynamics.
To effectively navigate these issues, it is essential to implement targeted recruitment strategies tailored to specific study types, such as:
Continuous education that highlights the unique demands of clinical trials in medical devices, coupled with fostering a collaborative environment, can inspire team members to excel. By leveraging bioaccess's expertise in study management, organizations can enhance team dynamics and ultimately improve study outcomes.
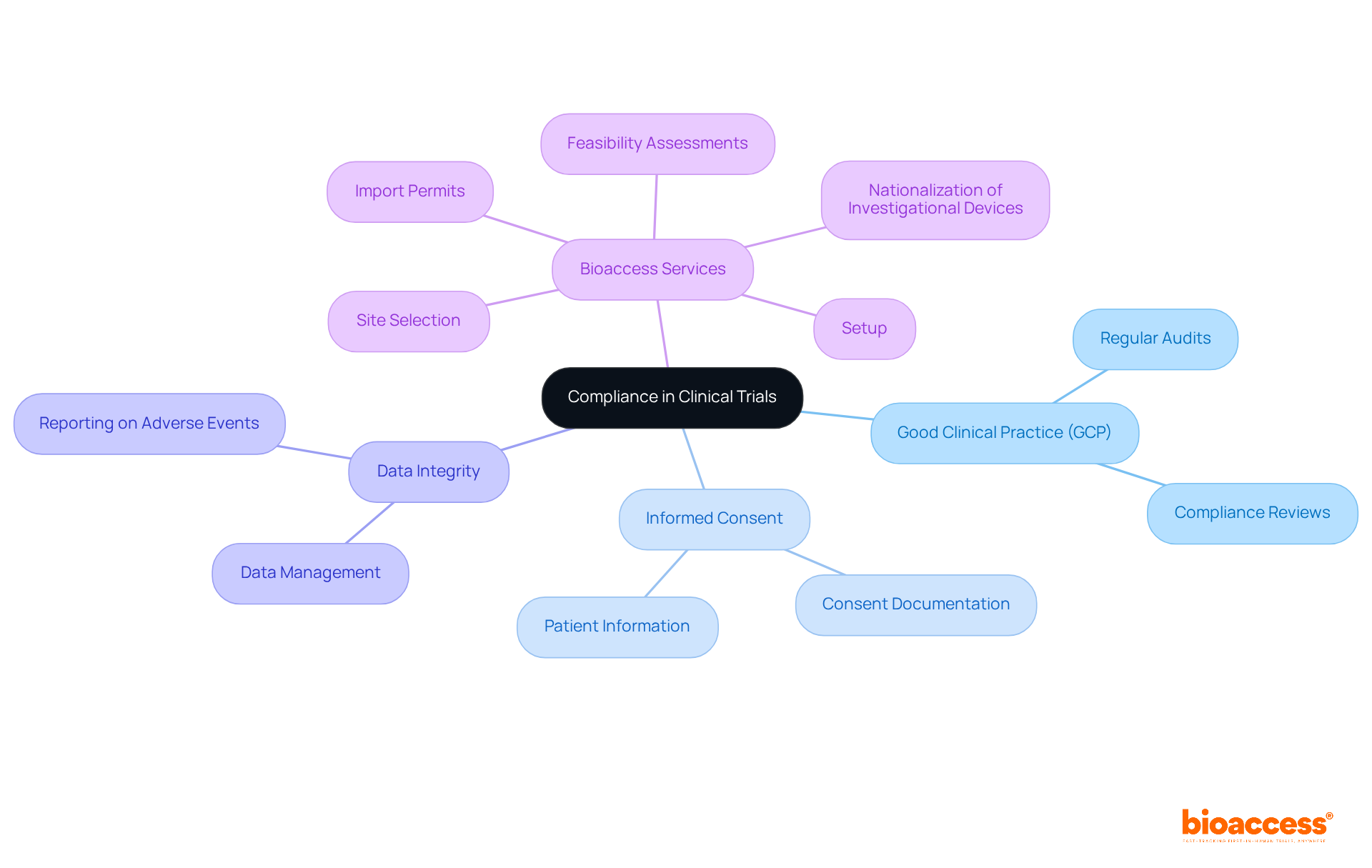
Adherence to regulatory standards is paramount in clinical research studies. This encompasses compliance with Good Clinical Practice (GCP) guidelines, ensuring informed consent, and safeguarding data integrity. Bioaccess offers comprehensive clinical research management services, which include:
By establishing robust compliance frameworks and conducting regular audits, research teams can mitigate risks and ensure their investigations uphold the highest ethical and scientific standards. Furthermore, effective project management and thorough reporting on study status and adverse events significantly enhance compliance and oversight throughout the research process.
Medical instruments possess the remarkable ability to significantly enhance patient outcomes by improving diagnosis, treatment, and monitoring. Innovations within this field pave the way for more effective therapies, reduced hospital stays, and an elevated quality of life for patients. Researchers can ensure that these devices are both safe and effective through rigorous clinical trials in medical devices, ultimately benefiting healthcare systems and patients alike.
To successfully promote medical technologies, Medtech firms must implement strategic methods for research studies. This includes leveraging bioaccess®'s expertise to achieve 50% faster patient enrollment and $25K savings through FDA-ready data, eliminating rework and delays. Bioaccess® specializes in managing various types of research, such as:
Identifying key performance indicators, utilizing data analytics for decision-making, and fostering collaborations with stakeholders across the healthcare ecosystem are essential components of this strategy.
By aligning clinical trials in medical devices with broader business objectives, companies can enhance their innovation pipeline and ensure successful market entry. Navigating the complexities of early-feasibility, first-in-human, and pivotal studies is crucial in this process. The integration of bioaccess®'s capabilities not only streamlines research but also positions firms to respond effectively to the dynamic Medtech landscape. As organizations strive for excellence, the emphasis on collaboration and strategic alignment will be pivotal in overcoming challenges and achieving sustainable growth.

The exploration of clinical trials for medical devices reveals a multifaceted approach that underscores the significance of strategic planning and execution. By leveraging regions with expedited regulatory processes, diverse patient demographics, and streamlined ethical approvals, organizations can notably enhance the efficiency and effectiveness of their research efforts. The central message emphasizes that adopting these strategies not only accelerates the timeline for bringing innovative medical devices to market but also improves patient outcomes through rigorous testing and validation.
Key insights from the article highlight the advantages of conducting clinical trials in Latin America, where regulatory speed and diverse patient pools can lead to faster approvals and more representative data. Furthermore, the role of bioaccess® in facilitating these processes through its expertise in early-phase studies and market access services illustrates the potential for Medtech companies to navigate complex regulatory landscapes successfully. This strategic alignment is crucial for optimizing research outcomes and achieving successful commercialization.
As the landscape of medical technology continues to evolve, it becomes increasingly vital for organizations to embrace these proven strategies. By prioritizing regulatory adherence, fostering diverse recruitment practices, and leveraging innovative technologies, companies can not only enhance their research capabilities but also contribute to the broader goal of improving patient care. The call to action is clear: to thrive in the competitive Medtech arena, stakeholders must commit to strategic collaboration and continuous improvement in clinical trial methodologies.
What is bioaccess® and what does it do?
bioaccess® is an organization that accelerates clinical trials for medical devices by leveraging regulatory efficiency in Latin America, diverse patient demographics in the Balkans, and effective ethical approval procedures in Australia.
How quickly can bioaccess® secure ethical approvals for clinical trials?
bioaccess® can secure ethical approvals in as little as 4-6 weeks.
How do enrollment rates for clinical trials conducted by bioaccess® compare to traditional markets?
Enrollment rates for clinical trials conducted by bioaccess® are 50% faster than those in traditional markets.
What notable success story highlights bioaccess®'s effectiveness?
A notable success is Mitralign, a Boston-based company that moved its medical study to Colombia, achieving ethical approval in 18 days and enrolling 11 participants within eight weeks, which accelerated its EU submission by 14 months.
What trends are influencing medical device studies as of 2025?
The integration of advanced technologies such as AI and machine learning is enhancing data collection and analysis in medical device studies.
How many regulatory submissions has bioaccess® completed since its inception?
Since its inception in 2010, bioaccess® has successfully completed over 159 regulatory submissions for more than 75 clinical trials in medical devices.
What is the significance of regulatory speed in Latin America for clinical trials?
Latin America's swift regulatory procedures significantly shorten the timeline from concept to commercialization for Medtech companies, facilitating faster market entry for innovative devices.
How long does it take for regulatory authority approval in Brazil?
Recent changes in Brazil's research approval process have reduced the average time to regulatory authority approval to approximately 51 days.
Why is diversity in patient pools important for clinical trials?
Diversity in patient pools enhances the validity of clinical trial results and provides insights into how different populations respond to medical devices, ultimately leading to improved patient outcomes.
What expertise does bioaccess® bring to managing clinical trials?
bioaccess® has over 20 years of experience in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies.