


The article identifies key therapeutic areas that are propelling clinical research forward, underscoring the significance of various medical fields in enhancing healthcare. It highlights that:
are essential for the development of innovative treatments and the improvement of patient outcomes. These advancements are further supported by technological progress and strategic partnerships.
The landscape of clinical research is rapidly evolving, driven by an urgent need to address diverse health challenges across various therapeutic areas. From the complexities of oncology and cardiovascular diseases to the rising prevalence of metabolic and neurological disorders, researchers are racing to develop innovative therapies that can significantly improve patient outcomes.
This article delves into ten key therapeutic areas that are not only shaping the future of clinical trials but also highlighting the critical role of organizations like bioaccess® in accelerating this vital research. As the demand for effective treatments grows, it is essential to explore the emerging trends and challenges that will define the next wave of clinical research.
bioaccess® leverages its unique geographical advantages to expedite oncology clinical trials in Colombia. By capitalizing on Latin America's regulatory efficiency, where ethical approvals can be secured in just 90-120 days, and the region's diverse populations, bioaccess® facilitates timely access to innovative therapies that significantly enhance survival rates.
Colombia's healthcare system, recognized among the best globally, guarantees high-quality care, while the country's universal healthcare coverage optimizes patient recruitment. Moreover, bioaccess® offers substantial financial benefits, with savings exceeding 30% compared to studies conducted in North America or Western Europe, complemented by R&D tax incentives that further enhance the attractiveness of conducting research in Colombia.
The partnership with Caribbean Health Group positions Barranquilla as a key hub for medical studies, thereby boosting the efficiency and effectiveness of oncology investigations. The organization’s expertise in managing complex studies across therapeutic areas, particularly oncology, allows researchers to focus on developing innovative therapies while ensuring compliance with regulatory standards.

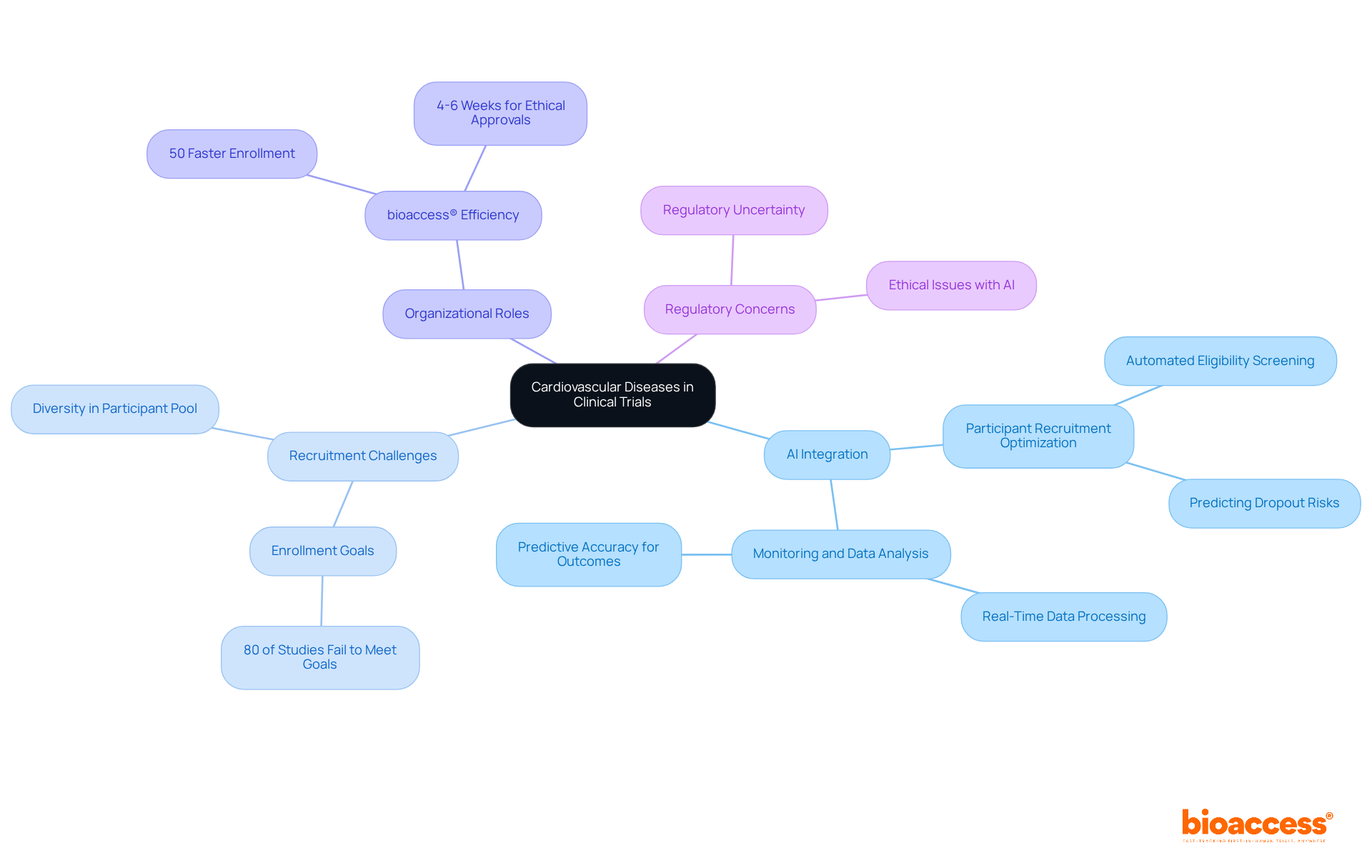
Cardiovascular illnesses remain a focal point in research studies within therapeutic areas, constituting a substantial portion of ongoing investigations aimed at evaluating innovative treatments to mitigate illness and mortality associated with heart disorders. The integration of artificial intelligence (AI) and data analytics into study designs is revolutionizing participant recruitment and monitoring processes, leading to more effective interventions.
For example, AI algorithms can optimize recruitment by automating eligibility screening and predicting dropout risks, thereby ensuring a diverse participant pool and enhancing retention rates. This technological advancement is crucial, considering that nearly 80% of medical studies fail to meet enrollment goals in a timely manner. Furthermore, AI has demonstrated the ability to predict hospitalizations for heart failure with over 75% accuracy, underscoring its potential in improving outcomes within cardiovascular research.
Organizations like bioaccess® play a vital role in facilitating these studies, connecting innovative Medtech, Biopharma, and Radiopharma startups with leading medical investigation sites. With the capacity to enroll treatment-naive cardiology groups 50% faster than their Western counterparts and secure ethical approvals in just 4-6 weeks, bioaccess® accelerates the medical research process, enabling innovators to bring their therapies to market more rapidly while saving $25,000 per patient.
As emphasized by authorities in therapeutic areas, particularly cardiovascular research, including the American Heart Association, the deployment of AI not only streamlines research operations but also enhances the predictive accuracy of health outcomes, ultimately transforming the landscape of cardiovascular disease management. However, it is essential to acknowledge the challenges posed by regulatory uncertainty and ethical concerns surrounding AI in medical studies, which remain significant factors in the current landscape.
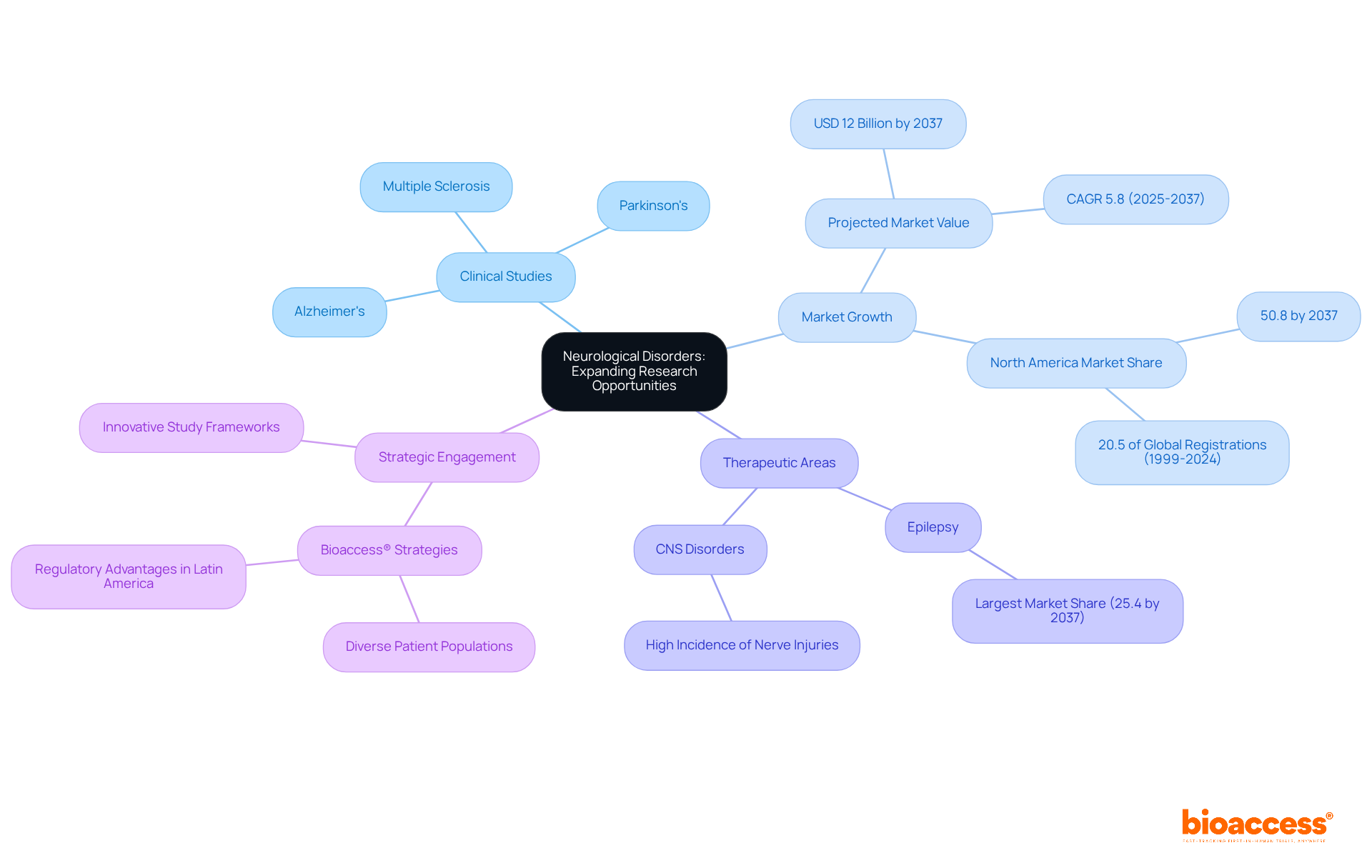
The landscape of neurological disorders is undergoing significant transformation, characterized by an increase in clinical studies across different therapeutic areas, including Alzheimer's, Parkinson's, and multiple sclerosis. The intricate nature of these disorders necessitates innovative study frameworks and effective participant engagement strategies. Bioaccess® is strategically positioned to enhance these studies, leveraging its diverse patient populations in the Balkans and the regulatory advantages of Latin America to expedite results. This dedication to addressing neurological disorders not only confronts urgent health challenges but also establishes a foundation for innovative therapies in therapeutic areas.
Recent statistics indicate that the neurology trial market is projected to reach USD 12 billion by 2037, growing at a CAGR of 5.8% from 2025 to 2037, underscoring the urgency and potential of this sector. Moreover, ongoing research is uncovering promising insights into Alzheimer's and Parkinson's, particularly emphasizing the repurposing of existing medications to hasten treatment availability. As the industry advances, bioaccess® remains at the forefront, championing the development of innovative solutions in neurology.
Infectious diseases have emerged as a critical focus in therapeutic areas of medical research, particularly in response to the challenges posed by global pandemics. The urgent need for effective vaccines and therapies has led to a significant increase in research studies focusing on various therapeutic areas related to pathogens.
bioaccess® is at the forefront of this initiative, streamlining study initiation and ensuring effective patient recruitment across diverse demographic areas. Our comprehensive management services for studies include:
Committed to ethical investigative practices, bioaccess® guarantees that all studies comply with the highest standards of safety and efficacy. Recent statistics reveal that approximately 1 in 10 vaccines aimed at emerging and reemerging viral infectious diseases (EVIDs) that have reached phase 2 since 2005 progressed to FDA approval within a decade, underscoring the significance of prompt and efficient studies. The organization’s strategic approach not only accelerates vaccine development timelines but also increases the likelihood of successful outcomes across therapeutic areas in the battle against infectious diseases.
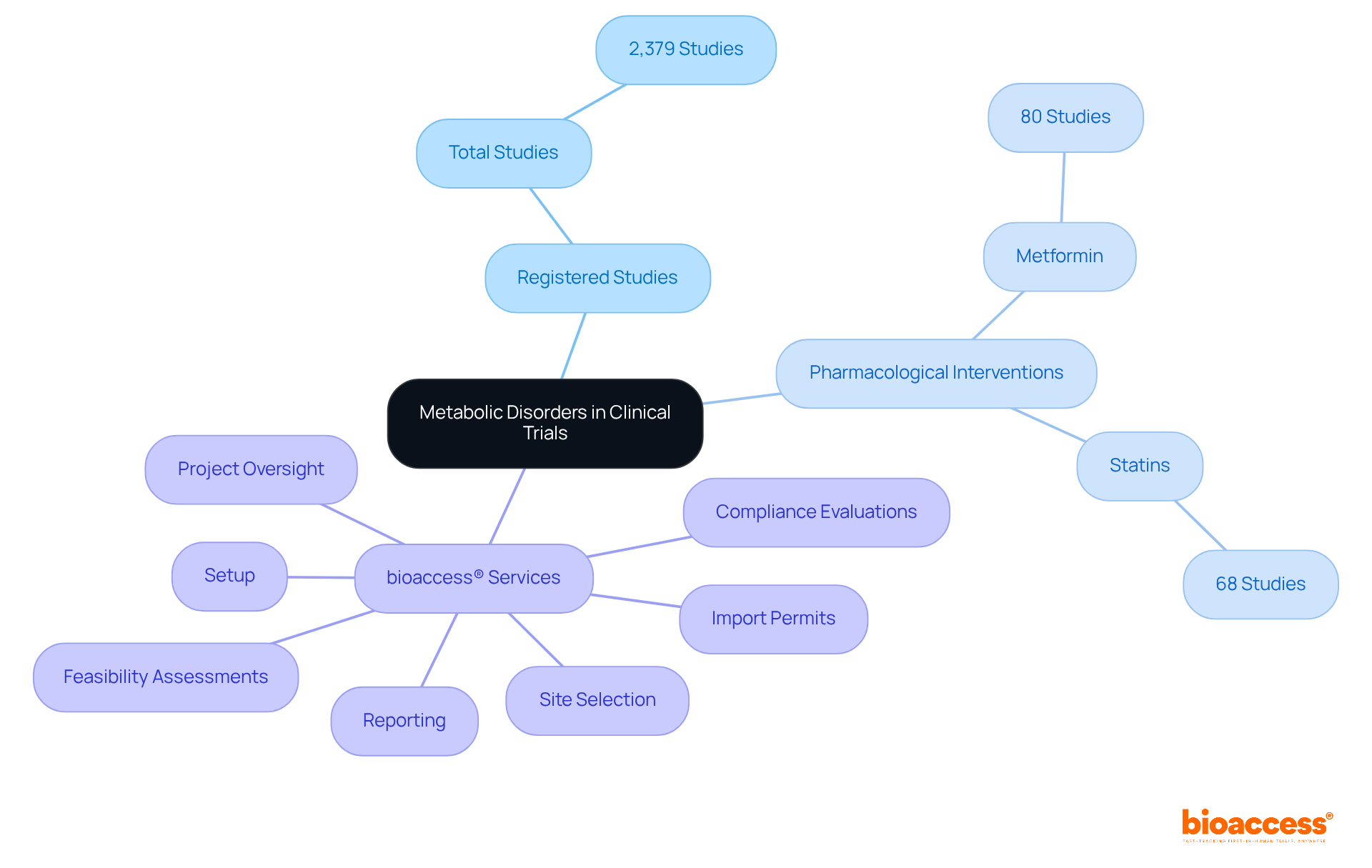
Metabolic disorders, particularly obesity and diabetes, are on the rise, necessitating urgent action from the medical community. As of April 2023, the International Clinical Trials Registry Platform documented 2,379 registered studies, highlighting a significant focus on therapeutic areas, particularly metabolic disorders. Among these, metformin and statins stand out as leading pharmacological interventions, with 80 and 68 studies, respectively.
In this critical arena, bioaccess® is at the forefront, leveraging its comprehensive study management services, which encompass:
All aimed at expediting timelines. The organization’s dedication to tackling these urgent health challenges is evident through its successful initiatives designed to assess new treatment options and enhance the understanding of disease mechanisms.
Current trends reveal an increasing focus on innovative therapies across therapeutic areas, with a marked rise in studies addressing metabolic syndrome, which impacts 61.6% of obese adults and 8.6% of those of normal weight. By conducting essential medical studies, bioaccess® significantly contributes to advancing research that can lead to effective solutions for obesity and diabetes. This effort is further bolstered by its collaboration with Caribbean Health Group to position Barranquilla as a premier site for medical studies in Latin America, a move supported by Colombia's Minister of Health.
Respiratory diseases, particularly asthma and chronic obstructive pulmonary disease (COPD), pose a significant global health challenge, affecting millions around the world. As we approach 2025, a notable increase in medical studies in therapeutic areas focused on these conditions is anticipated, reflecting a growing awareness of their impact on public health. In this context, bioaccess® plays a pivotal role by facilitating access for diverse populations across Latin America, the Balkans, and Australia, which is essential for robust clinical studies. The organization ensures compliance with regulatory standards, thereby streamlining testing processes for greater efficiency.
Recent advancements in therapeutic areas for asthma and COPD are promising, as innovative treatments are being developed to specifically target the underlying mechanisms of these diseases. Current research trends emphasize personalized medicine approaches across therapeutic areas, aiming to tailor treatments to individual profiles for enhanced outcomes. Successful initiatives, including collaborative studies and community engagement programs, have demonstrated their effectiveness in improving participant recruitment and retention in therapeutic areas, ultimately accelerating the development of effective treatments.
As the prevalence of asthma and COPD continues to escalate, the dedication of organizations like bioaccess® to advancing respiratory health across therapeutic areas through clinical trials becomes increasingly vital. Their efforts not only deepen scientific understanding of these diseases but also pave the way for new treatment options that can significantly improve the quality of life for individuals.
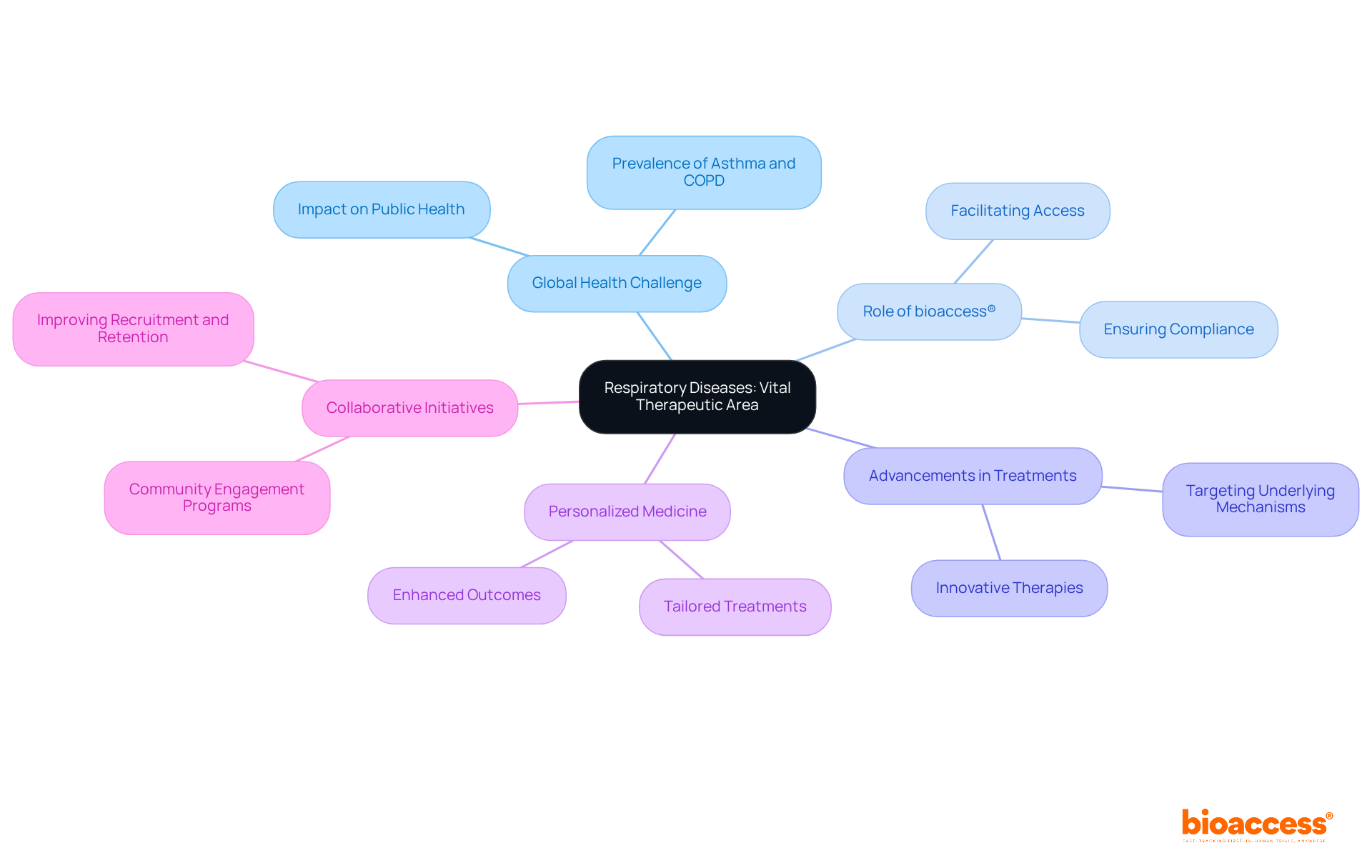
Gastrointestinal disorders, particularly inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), are increasingly recognized in therapeutic areas for their prevalence and debilitating effects on individuals. Recent estimates indicate that IBD affects approximately 2.4 to 3.1 million adults in the U.S., with the highest prevalence among non-Hispanic White populations at 0.8%. The incidence of IBS has surged by 28% from 2017 to 2023, underscoring the urgent need for effective treatments and interventions in therapeutic areas.
Clinical trials are essential in evaluating new therapies and elucidating the underlying mechanisms of these diseases. By 2025, trends in research suggest a growing focus on innovative treatment methods in therapeutic areas, including biologics and dietary interventions, which have shown promise in improving outcomes for patients. Successful initiatives, such as machine-learning classifiers, have achieved over 95% accuracy in categorizing regions based on IBD epidemiology, thereby enhancing our understanding of disease patterns.
bioaccess® stands at the forefront of gastrointestinal studies, leveraging its regulatory expertise and access to diverse populations in Latin America, the Balkans, and Australia. By facilitating prompt and efficient studies, bioaccess® is committed to advancing healthcare solutions for individuals affected by IBD and IBS, ensuring that groundbreaking treatments reach the market more swiftly and effectively.
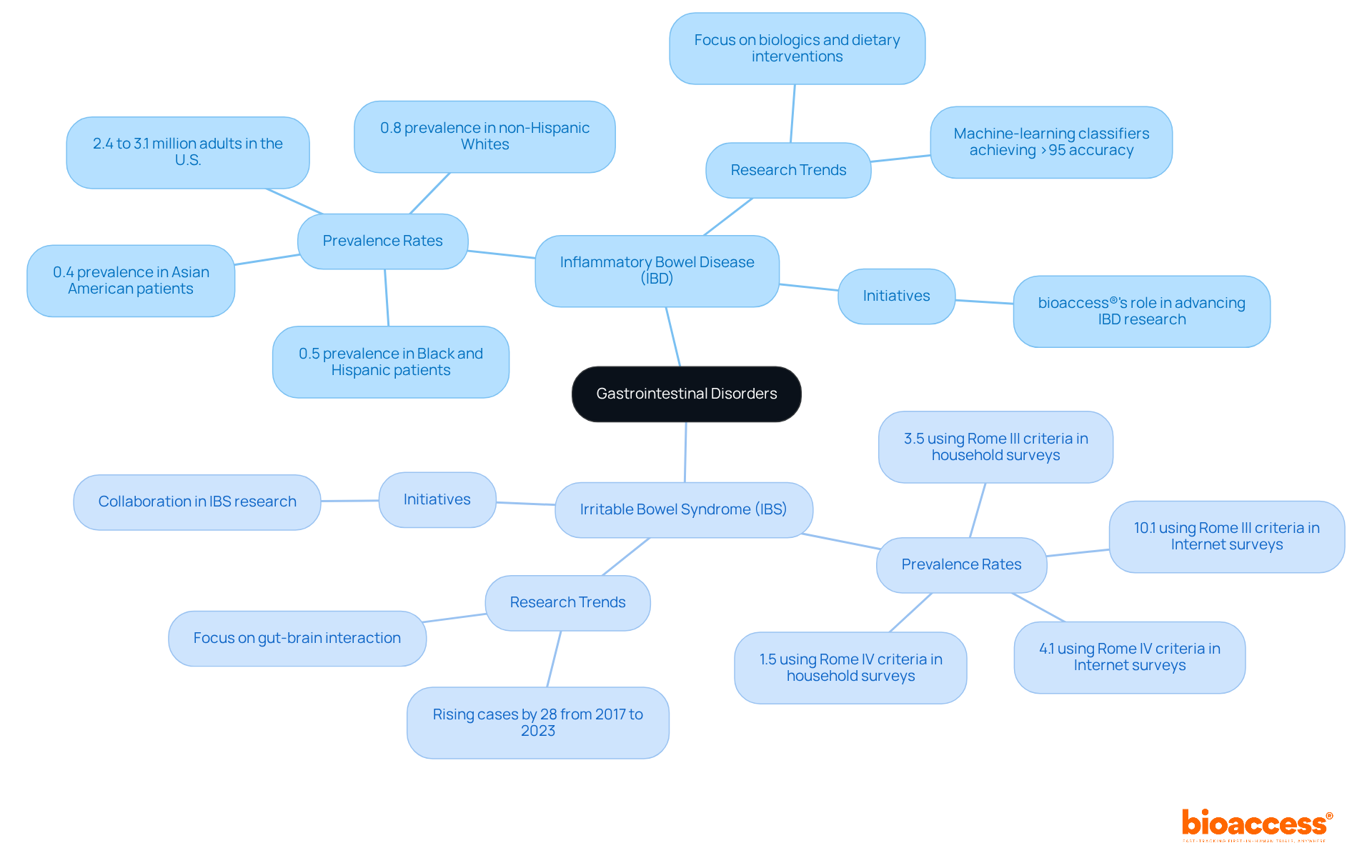
Dermatological issues, particularly psoriasis and eczema, are increasingly recognized in medical studies due to their profound impact on individuals' quality of life. By 2025, the number of research studies focusing on these conditions is expected to rise significantly, reflecting a growing commitment to developing innovative treatments. Recent analyses indicate that skin disorders rank fourth in the global burden of disease concerning years lived with disability, highlighting the urgent need for effective therapies.
bioaccess® is pivotal in advancing dermatological research by ensuring access to diverse patient populations across Latin America, the Balkans, and Australia. Our comprehensive research study management services encompass:
This strategic approach not only accelerates enrollment but also enhances the inclusivity of research studies, addressing the historical underrepresentation of minority groups in dermatology investigations. For instance, a recent initiative successfully recruited over 300 non-Hispanic Black men for a research study, achieving an impressive retention rate of 95%.
Successful research initiatives in psoriasis and eczema are paving the way for groundbreaking treatments. The FDA's recent draft guidance underscores the importance of diversity in research trials, aiming to boost participation from underrepresented groups. This aligns with bioaccess®'s mission to ensure that clinical studies accurately reflect the demographics of the populations they intend to serve.
Furthermore, advancements in treatment options, including promising results from studies on biologics and novel therapies, are anticipated to significantly improve patient outcomes. Psoriasis and atopic dermatitis exhibit a global prevalence ranging from 0.5% to 5% in adults, underscoring the importance of these conditions in the global health landscape. As the realm of dermatological studies evolves, bioaccess® remains committed to supporting these efforts, ensuring compliance with regulatory standards while fostering innovation in the field.
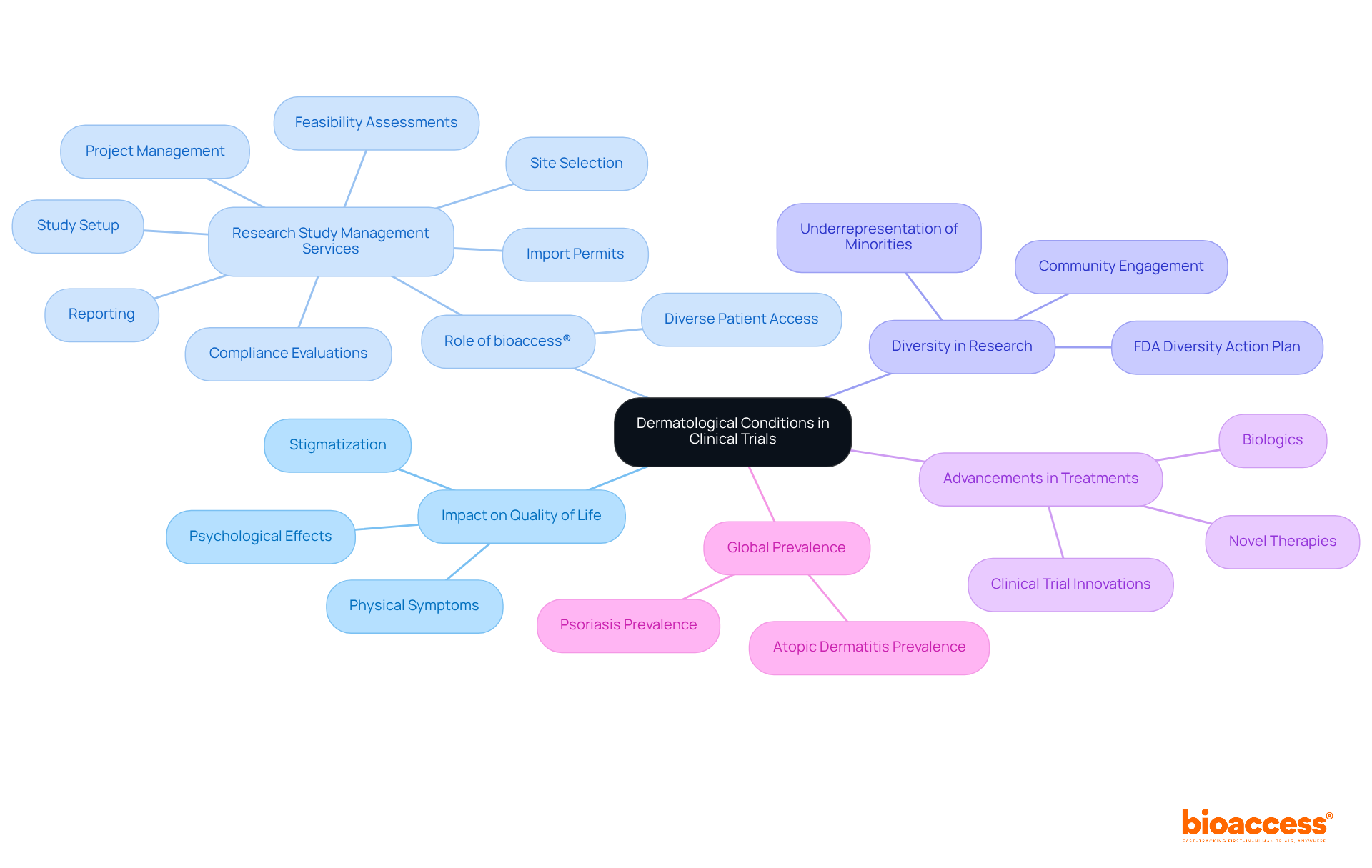
Women's health has emerged as a critical focus in medical research, particularly in therapeutic areas like reproductive health, menopause, and autoimmune diseases. Recent studies reveal that nearly 15 million women in the U.S. were prescribed estrogen-based treatments in the 1990s, highlighting the historical dependence on hormone replacement therapy (HRT) for managing menopausal symptoms. However, following the 2002 Women's Health Initiative study, which raised concerns regarding the risks associated with HRT, its usage declined significantly, despite subsequent studies indicating that HRT can be beneficial for women within ten years of menopause.
The prevalence of reproductive health issues warrants attention, with conditions like endometriosis affecting approximately 10% of women of reproductive age. Moreover, a 2023 study underscored that declining estrogen levels during menopause elevate the risk of cardiovascular disease, the leading cause of death for both genders in the U.S. This growing body of evidence emphasizes the urgent need for research in therapeutic areas that specifically target women's health to develop effective, tailored treatments. Additionally, women spend 25% more of their lives in debilitating health compared to men, underscoring the critical importance of addressing these challenges.
bioaccess® plays a pivotal role in advancing women's health research by leveraging its comprehensive study management services, which include:
The organization ensures adherence to regulatory standards while expediting the research process, thereby fostering innovative solutions that address the unique health issues faced by women. The global menopause market is projected to expand from $17.66 billion in 2024 to $27.63 billion by 2033, reflecting a growing demand for menopause-related health solutions. By prioritizing women's health, bioaccess® is dedicated to improving healthcare outcomes and propelling the development of effective treatments tailored to women's needs. Furthermore, over 90% of residency program directors acknowledge the necessity for a standardized menopause curriculum, which bioaccess® could help fulfill through its initiatives.
Pediatric studies represent a critical component of medical research, particularly in the therapeutic areas that address the unique physiological and developmental needs of children, which differ significantly from those of adults. Clinical studies focused on therapeutic areas for pediatric populations are vital to ensuring the safety and efficacy of treatments tailored for younger individuals. In 2023, it was noted that fewer than 20% of medical studies included young participants, underscoring the urgent need for more focused research in this domain.
Bioaccess® is at the forefront of pediatric studies, leveraging its regulatory expertise and extensive patient access capabilities to facilitate swift and effective research. The organization’s commitment to advancing therapeutic areas, particularly in pediatric health through research, is essential for tackling the specific challenges faced by this demographic.
Recent trends reveal an increasing focus on targeted therapies within therapeutic areas for pediatric conditions, particularly in oncology, where it is estimated that 9,620 children in the U.S. will be diagnosed with cancer in 2024. Successful initiatives, such as the collaboration between Pfizer and Valneva SE on the Phase 3 study of the Lyme disease vaccine candidate VLA15, underscore the importance of innovative research in therapeutic areas to improve health outcomes for children.
As the pediatric clinical trials market evolves, bioaccess® remains steadfast in its mission to enhance pediatric health research in multiple therapeutic areas, ensuring that children receive the safe and effective treatments they rightfully deserve.
The exploration of key therapeutic areas driving clinical research forward reveals a dynamic landscape of medical studies aimed at addressing critical health challenges. Focusing on areas such as:
underscores the importance of innovative solutions and timely access to effective treatments.
Throughout this article, various insights highlight the role of organizations like bioaccess® in accelerating clinical trials. By leveraging regulatory advantages in Colombia and integrating advanced technologies like AI in participant recruitment, these strategies enhance the efficiency and effectiveness of research. The commitment to addressing urgent health needs, improving patient outcomes, and fostering diversity in clinical trials reflects a broader trend towards more inclusive and impactful medical research.
As the field continues to evolve, the imperative for ongoing investment and focus in these therapeutic areas cannot be overstated. Stakeholders in healthcare and research are encouraged to prioritize collaboration and innovation to ensure that advancements in clinical trials translate into real-world benefits for patients. Emphasizing the significance of these efforts will not only improve health outcomes but also pave the way for groundbreaking treatments that can change lives.
What is bioaccess® and how does it contribute to oncology clinical trials?
bioaccess® accelerates oncology clinical trials in Colombia by leveraging the region's regulatory efficiency, allowing ethical approvals within 90-120 days, and utilizing diverse populations to provide timely access to innovative therapies that enhance survival rates.
What advantages does Colombia offer for conducting clinical trials?
Colombia's healthcare system is recognized globally for its high-quality care and universal healthcare coverage, which optimizes patient recruitment. Additionally, conducting studies in Colombia can lead to financial savings exceeding 30% compared to North America or Western Europe, along with R&D tax incentives.
How does bioaccess® enhance the efficiency of oncology investigations?
bioaccess® partners with Caribbean Health Group to establish Barranquilla as a key hub for medical studies, facilitating the management of complex oncology studies while ensuring compliance with regulatory standards, allowing researchers to focus on developing innovative therapies.
What role does artificial intelligence (AI) play in cardiovascular disease clinical trials?
AI and data analytics are revolutionizing participant recruitment and monitoring in cardiovascular research, optimizing eligibility screening and predicting dropout risks, which enhances the diversity of participant pools and retention rates.
What challenges do clinical studies in cardiovascular diseases face?
Nearly 80% of medical studies fail to meet enrollment goals in a timely manner, and there are significant challenges posed by regulatory uncertainty and ethical concerns surrounding the use of AI in medical studies.
How does bioaccess® support cardiovascular disease research?
bioaccess® connects innovative Medtech, Biopharma, and Radiopharma startups with leading medical investigation sites, enabling them to enroll treatment-naive cardiology groups 50% faster than Western counterparts and secure ethical approvals in just 4-6 weeks.
What is the projected market growth for neurological disorder trials?
The neurology trial market is projected to reach USD 12 billion by 2037, growing at a CAGR of 5.8% from 2025 to 2037, highlighting the urgency and potential in this sector.
How is bioaccess® positioned to enhance studies on neurological disorders?
bioaccess® leverages diverse patient populations in the Balkans and the regulatory advantages of Latin America to expedite results in clinical studies for neurological disorders, including Alzheimer's, Parkinson's, and multiple sclerosis.