


The article articulates the advantages of selecting a contract research lab for clinical trials, highlighting key benefits such as:
These elements are essential as they significantly enhance the efficiency and success rates of clinical research. By leveraging these advantages, companies can bring innovations to market more rapidly and effectively, addressing the pressing challenges within the Medtech landscape.
The landscape of clinical trials is evolving at an unprecedented pace, with contract research organizations (CROs) emerging as pivotal players in enhancing both efficiency and effectiveness. By leveraging specialized expertise and agile methodologies, these organizations provide a unique opportunity for Medtech and Biopharma companies to navigate complex regulatory environments and expedite their research processes.
However, with a plethora of options available, what truly distinguishes a contract research lab in delivering successful clinical outcomes? This article explores ten compelling reasons to choose a CRO, examining how these partnerships can not only streamline trials but also drive innovation and improve patient care.
This company stands out in the medical field by offering unparalleled flexibility, enabling pioneers in Medtech, Biopharma, and Radiopharma to expedite their trials. By focusing on early-phase studies—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—the system merges regulatory speed, achieving approvals in just 6-8 weeks, with diverse patient populations. This ensures that clinical research is not only efficient but also effective. Such agility allows clients to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, translating to substantial cost savings of $25K per patient, all while providing FDA-ready data—no rework, no delays. Ultimately, this solution empowers clients to accelerate their innovations to market, enhancing patient outcomes and advancing medical knowledge.

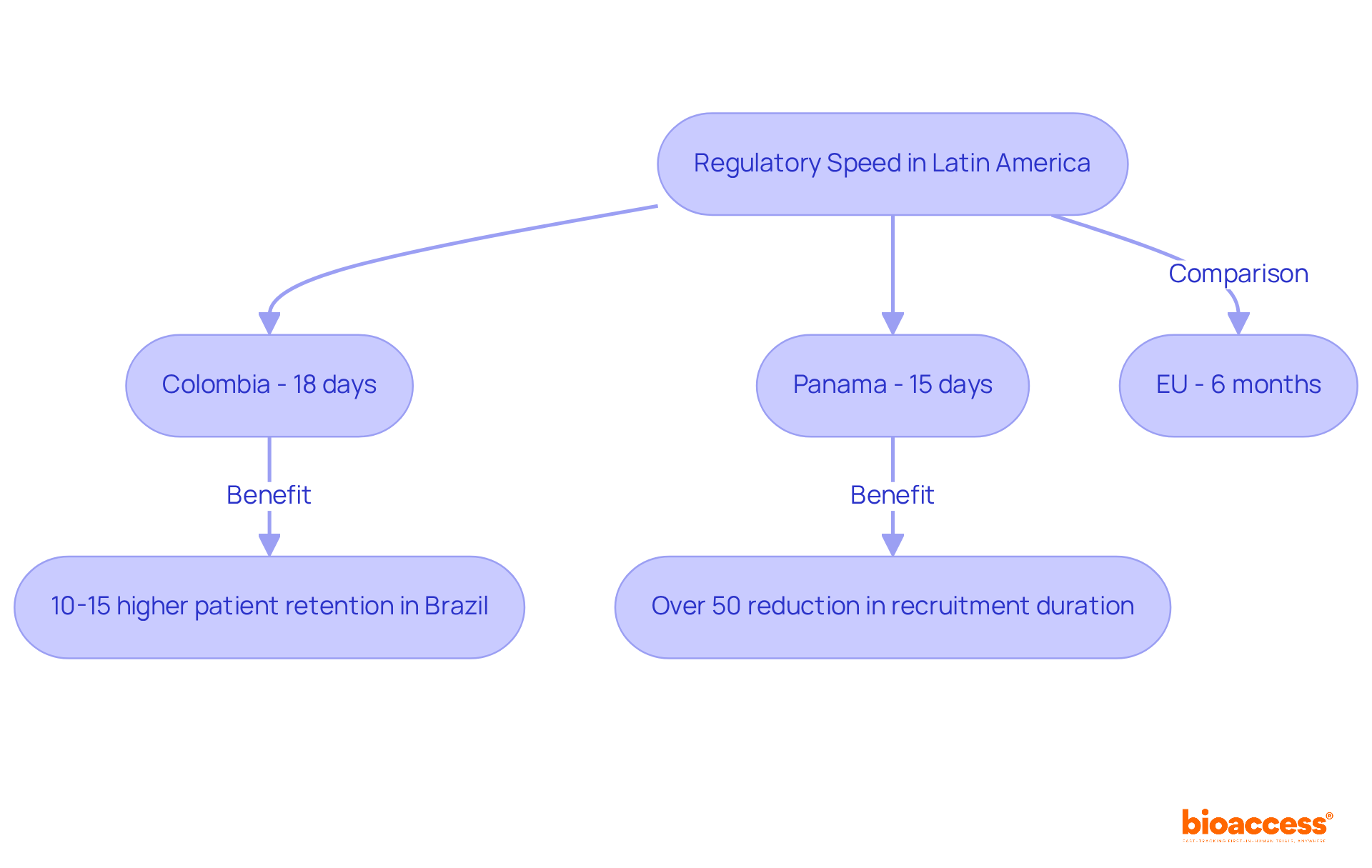
Latin America is distinguished by its exceptional regulatory speed, allowing bioaccess® to secure ethical approvals in an impressive 4 to 6 weeks. This rapid response is crucial for companies aiming to minimize delays in their clinical studies. For example, Mitralign achieved ethical approval in just 18 days in Colombia, a significant acceleration compared to the typical six-month timeline in the EU. Similarly, Newrotex obtained authorization in Panama within 15 days for its nerve regeneration technology study. Such expedited processes enable innovators to focus on their research and development efforts without the extensive waiting periods often faced in traditional markets.
Experts emphasize that the swift approval timelines in Latin America, particularly in Brazil and Colombia, provide a competitive edge for research studies. Brazil's patient retention rates are notably 10-15% higher than global averages, enhancing the effectiveness of execution. By leveraging these fast-track approval processes, companies can navigate the complexities of medical research more efficiently, ensuring timely access to new therapies and innovations.
Recent successes, such as ReGelTec's Early Feasibility Study on HYDRAFIL™ for managing chronic low back pain, illustrate the potential of conducting studies in Colombia. The collaboration between the organization and Caribbean Health Group positions Barranquilla as a leading site for research studies in Latin America, supported by Colombia's Minister of Health. Additionally, GlobalCare Clinical Studies' partnership with another organization has enhanced ambulatory services for research in Colombia, leading to over a 50% reduction in recruitment duration and 95% retention rates. As Dushyanth Surakanti, CEO of Sparta Biomedical, notes, collaborating with this product during its initial human study in Colombia has highlighted the unique advantages that Latin America offers for medical exploration efforts.
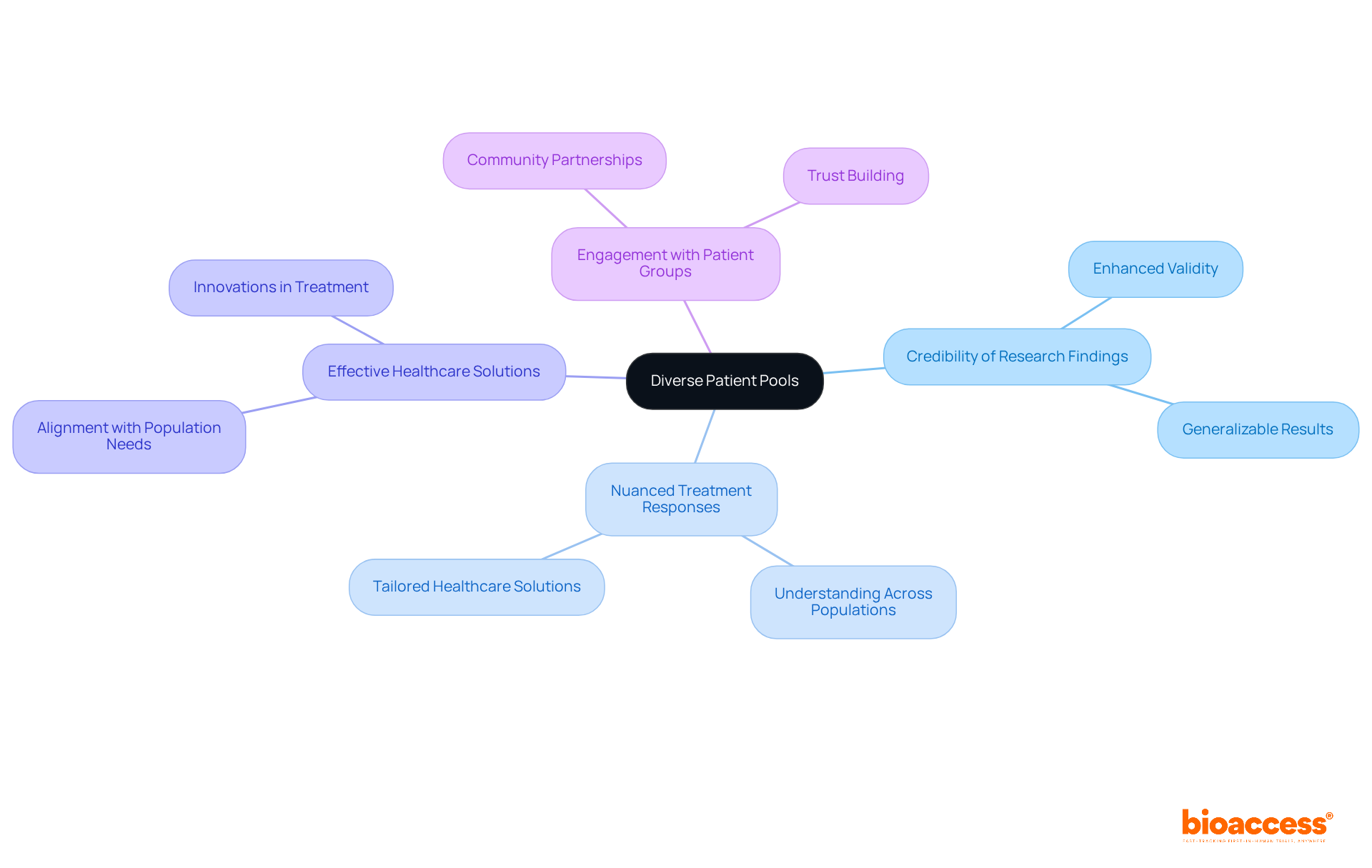
The Balkans present a unique opportunity for clinical trials, characterized by a rich diversity of patient demographics that significantly enhances the credibility of research findings. By capitalizing on this varied patient pool, bioaccess® can offer a more nuanced understanding of treatment responses across different populations. This inclusivity not only cultivates more effective healthcare solutions but also guarantees that clinical research aligns with the broader population's needs.
Experts in the field emphasize that enhancing study validity through demographic diversity is crucial for generating generalizable results. By engaging with diverse patient groups, researchers can capture a wider array of responses, ultimately driving innovations that are representative and beneficial for all segments of society.
Early-phase studies, particularly first-in-human experiments, are vital for assessing the safety and efficacy of new medical interventions. These studies lay the groundwork for subsequent phases, providing essential insights that guide further development. Given that approximately 80% of clinical studies fail to achieve their initial enrollment goals, meticulous planning and execution of early-phase research become paramount.
bioaccess® distinguishes itself in this arena as a contract research lab, leveraging over 15 years of expertise to conduct studies effectively and ethically while ensuring cost-effective solutions for Medtech and Biopharma startups. This emphasis not only expedites the development process, achieving regulatory approval in just 6-8 weeks, but also significantly enhances the probability of success (POS) in later phases.
Successful first-in-human studies have demonstrated substantial advancements across various therapeutic areas, underscoring their critical role in the drug development pipeline. As industry experts emphasize, the methodologies employed in these studies are crucial for establishing a robust evidence base, ultimately leading to improved patient outcomes and expedited access to innovative therapies.

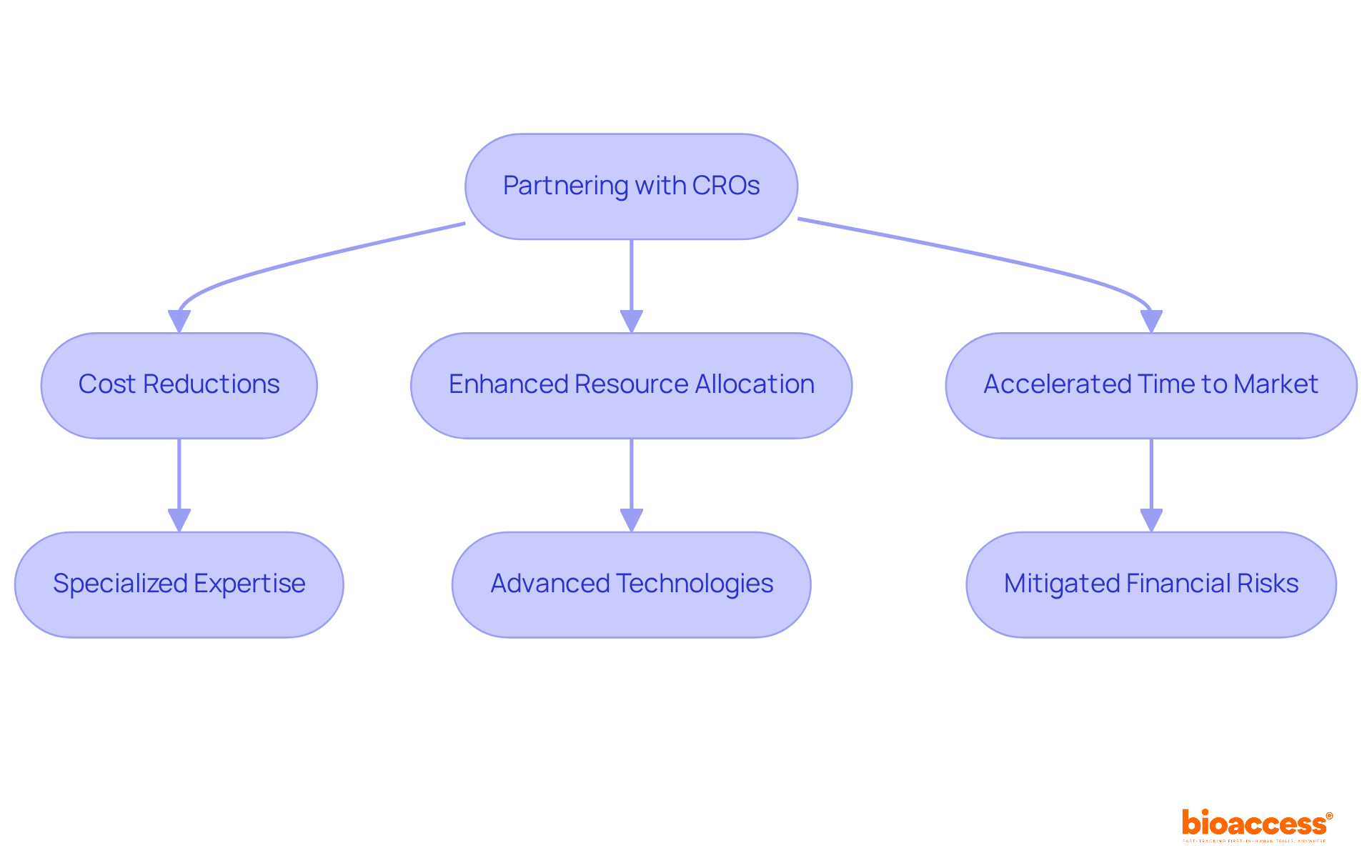
Partnering with a contract organization can yield substantial cost reductions for Medtech and Biopharma firms. By outsourcing research studies, these organizations can enhance their budgets and allocate resources more strategically. With a commitment to delivering high-quality services at competitive rates, the company ensures that clients achieve maximum value without compromising research quality. The global research outsourcing market is projected to reach USD 86.81 billion by 2033, underscoring the growing trend of companies leveraging CROs to enhance financial efficiency.
Through the utilization of specialized expertise and advanced technologies, CROs streamline processes, reduce operational costs, and ultimately accelerate the time to market for innovative therapies. For instance, firms that have collaborated with a specific partner have reported significant reductions in testing costs while maintaining rigorous standards of adherence and data integrity. This strategic approach not only mitigates financial risks but also empowers organizations to concentrate on their core competencies, thereby driving innovation in the healthcare landscape.
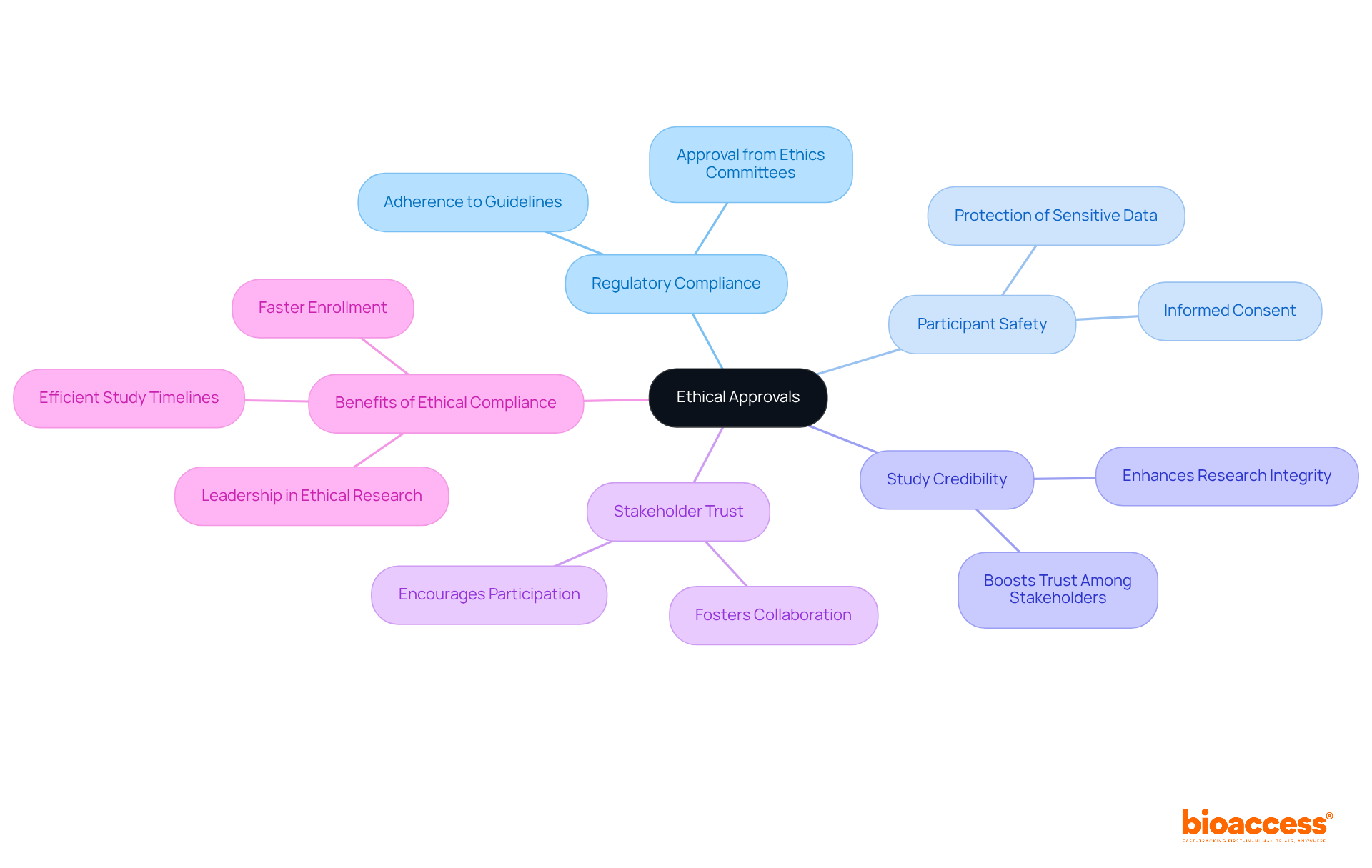
bioaccess® places a strong emphasis on ethical compliance across all clinical trials, underscoring its relevance in the Medtech landscape. By implementing a streamlined process for obtaining ethical approvals, the organization ensures that studies adhere to regulatory guidelines, which is crucial for maintaining participant safety and integrity. This commitment not only protects participants but also significantly boosts the credibility of the study, fostering trust among stakeholders.
Furthermore, swift ethical approvals can lead to faster enrollment and more efficient study timelines, ultimately benefiting the advancement of medical innovations. Regulatory agencies consistently emphasize the importance of ethical supervision, as it is fundamental to responsible inquiry practices. By prioritizing ethical standards, this organization not only meets but exceeds industry expectations, positioning itself as a leader in ethical clinical research.
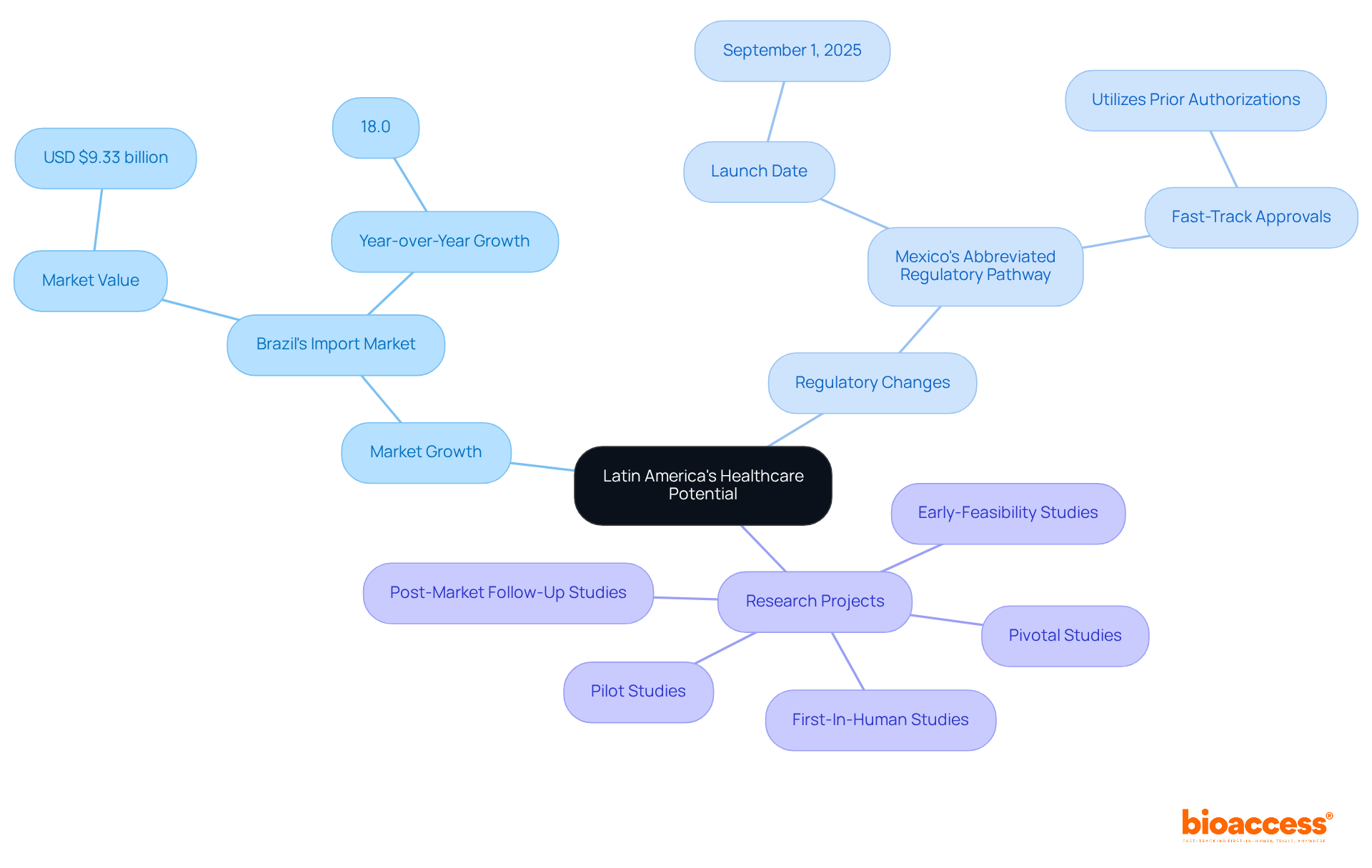
Latin America is rapidly emerging as a multi-billion dollar healthcare market, teeming with opportunities for innovative products. In 2024, Brazil's medical technology import market surged to USD $9.33 billion, marking an impressive 18.0% year-over-year increase in demand for advanced healthcare solutions. This significant growth underscores the region's potential for successful product commercialization.
Furthermore, Mexico's new Abbreviated Regulatory Pathway, set to launch on September 1, 2025, is anticipated to establish the country as a priority launch market for high-value medical devices, thereby enhancing market access in the region. The solution plays a pivotal role in assisting clients in navigating the complexities of market access, ensuring their products efficiently reach the right audiences.
By leveraging local expertise and regulatory knowledge, the company empowers organizations to capitalize on this lucrative market, facilitating swift and effective commercialization strategies. This organization is dedicated to overseeing diverse research projects, including:
As the healthcare landscape continues to evolve, the ability to navigate these opportunities will be crucial for innovators aiming to make a significant impact in Latin America.
The regulatory environment poses significant challenges for clinical study sponsors, often appearing intricate and intimidating. 'bioaccess®' offers unparalleled expertise in regulatory navigation, ensuring that studies proceed smoothly and efficiently. Our comprehensive process includes:
All tailored to meet specific country requirements. By conducting thorough reviews and providing feedback on study documents, we guarantee compliance with ethics committee and health ministry regulations. Moreover, we manage trial setup, import permits, and the nationalization of investigational devices, allowing clients to focus on their objectives while minimizing the risk of delays.

Engaging with a contract research lab such as bioaccess™ creates an environment where innovation thrives. This partnership model allows for tailored solutions that align with the unique objectives of each client, ensuring that specific needs are addressed throughout the research process. Such close collaboration not only enhances the quality of medical research but also accelerates the development of transformative therapies.
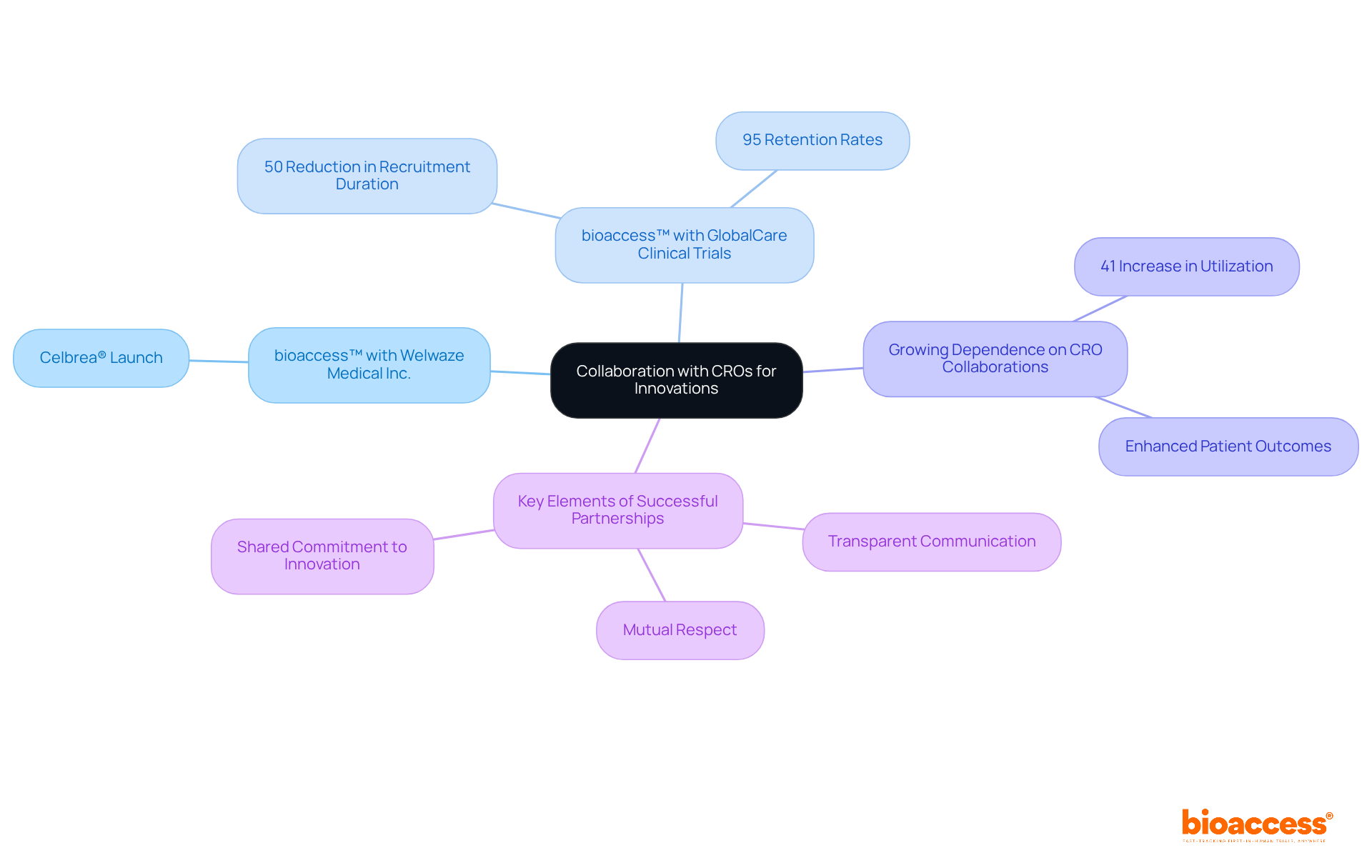
For example, bioaccess™ successfully collaborated with Welwaze Medical Inc. to facilitate the launch of the Celbrea® medical device in Colombia, demonstrating its proficiency in regulatory access and market entry. Additionally, partnerships with GlobalCare Clinical Trials have led to significant improvements in ambulatory services for studies, achieving over a 50% reduction in recruitment duration and 95% retention rates, which highlights the effectiveness of these strategic alliances.
Testimonials from industry leaders, including Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, further illustrate the positive impact of bioaccess™ during its inaugural human study in Colombia. These strategic alliances exemplify how effective partnerships can streamline processes and enhance patient outcomes.
Recent data indicates that 41% of drug developers have increased their utilization of contract research lab partnerships compared to two years ago, emphasizing the growing dependence on CRO collaborations. Industry leaders assert that successful collaborations hinge on transparent communication, mutual respect, and a shared commitment to innovation, ultimately driving the success of medical studies and enhancing the overall healthcare landscape.
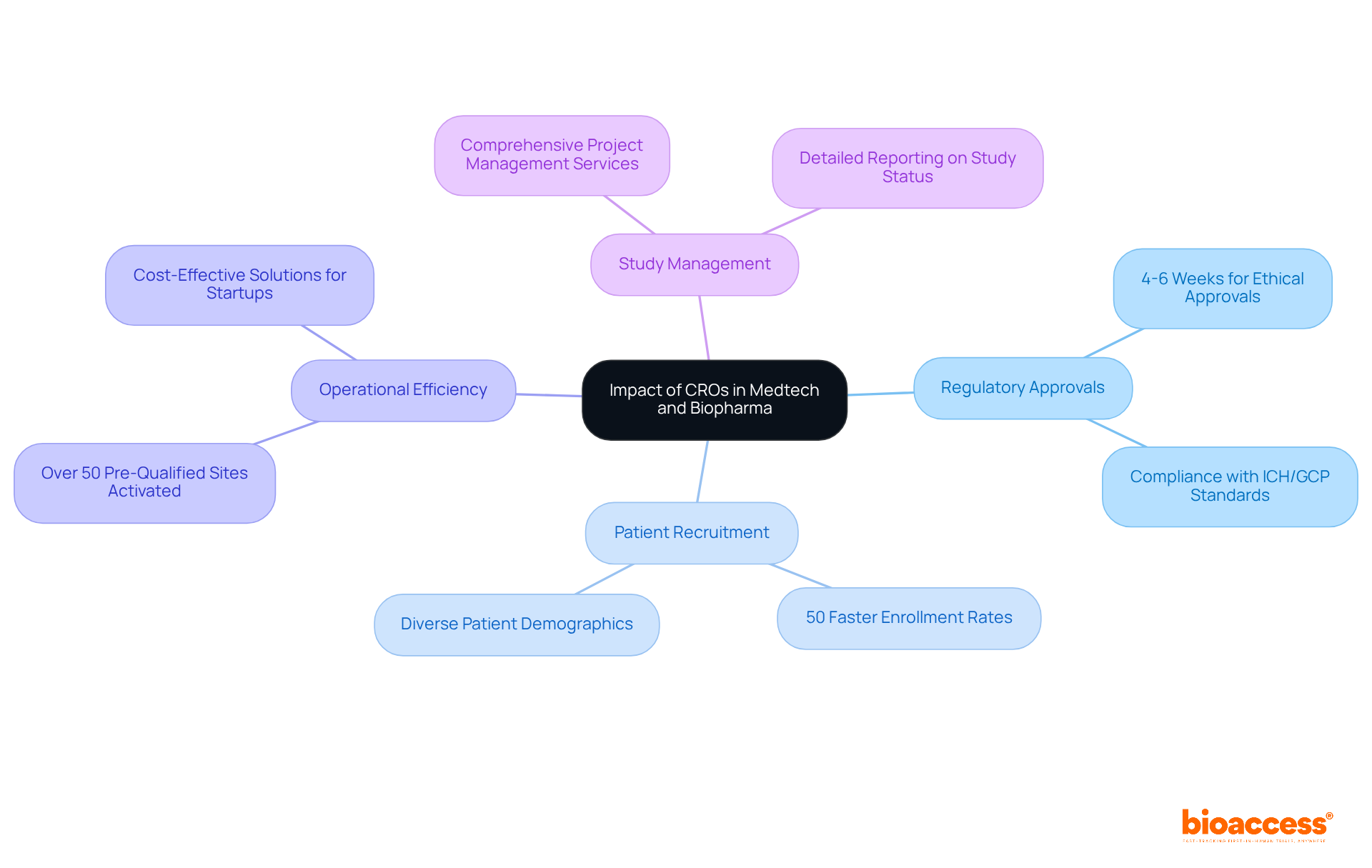
Contract research labs are pivotal to the success of Medtech and Biopharma firms, providing specialized expertise and resources that streamline the complexities of clinical studies. By leveraging their extensive knowledge of regulatory environments, CROs facilitate rapid ethical approvals, typically within 4 to 6 weeks, and enhance patient recruitment efficiency, achieving enrollment rates that are 50% faster than traditional markets. This capability is particularly crucial, as approximately 80% of medical studies face delays or termination due to recruitment challenges.
The contributions of CROs extend beyond individual studies; they are instrumental in advancing medical technology and improving patient outcomes across the industry. For example, the company has activated over 50 pre-qualified sites in less than eight weeks across Latin America, the Balkans, and Australia, demonstrating its ability to enhance operational efficiency and accelerate market entry for innovative medical technologies. Furthermore, bioaccess® provides comprehensive reporting on study status and adverse events, ensuring compliance and transparency throughout the trial process.
First-in-human (FIH) and early-feasibility studies are essential for gathering critical safety and effectiveness data, with about 70% of medications in these evaluations progressing to subsequent phases. Bioaccess® supports these initiatives by offering comprehensive project management services, including feasibility assessments, project oversight, and detailed reporting, which are vital for navigating the complexities of medical studies.
Industry analysts recognize the transformative impact of contract research labs on medical investigations, noting that their strategic collaborations and local knowledge significantly enhance the quality and inclusiveness of studies. By prioritizing patient diversity and adhering to regulatory standards, the organization not only safeguards research integrity but also contributes to more equitable healthcare outcomes. This commitment to ethical practices and operational excellence positions bioaccess® as a leader in the contract research lab sector, empowering Medtech and Biopharma innovators to effectively achieve their clinical trial objectives.
Choosing a contract research lab for clinical trials provides a strategic advantage that significantly enhances the efficiency and effectiveness of medical research. By leveraging the unique capabilities of organizations like bioaccess®, innovators in Medtech, Biopharma, and Radiopharma can adeptly navigate complex regulatory landscapes, expedite approvals, and access diverse patient populations. This partnership not only accelerates the journey from concept to market but also ensures that clinical research is grounded in ethical practices and robust methodologies.
The article underscores several compelling reasons for selecting a contract research organization (CRO), including:
Each of these factors contributes to a more streamlined research process that can reduce costs, enhance study validity, and ultimately lead to better patient outcomes. Furthermore, the emphasis on collaboration with CROs fosters innovation and drives success in clinical trials, ensuring that new therapies reach the market more efficiently.
In conclusion, the integration of CRO services is essential for any organization aiming to make a meaningful impact in the healthcare sector. By capitalizing on the advantages offered by contract research labs, stakeholders can optimize their clinical trials while contributing to the advancement of medical knowledge and improved patient care. Embracing this approach is a pivotal step toward transforming the future of healthcare, making it imperative for innovators to consider partnering with CROs to unlock their full potential in clinical research.
What is bioaccess® and how does it contribute to clinical research?
bioaccess® is a company that offers unparalleled flexibility in the medical field, enabling pioneers in Medtech, Biopharma, and Radiopharma to expedite their clinical trials, particularly in early-phase studies. It merges regulatory speed with diverse patient populations, achieving approvals in just 6-8 weeks and allowing clients to enroll treatment-naive cohorts 50% faster than Western sites.
What types of studies does bioaccess® focus on?
bioaccess® focuses on early-phase studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
How does bioaccess® ensure cost-effectiveness in clinical trials?
By providing FDA-ready data without the need for rework or delays, bioaccess® allows for substantial cost savings of $25K per patient, thereby enabling clients to accelerate their innovations to market.
What advantages does Latin America offer for clinical research?
Latin America, particularly countries like Colombia and Brazil, offers exceptional regulatory speed, allowing for ethical approvals in 4 to 6 weeks. This rapid response minimizes delays in clinical studies and provides a competitive edge for research.
Can you provide examples of successful studies in Latin America?
Yes, for instance, Mitralign achieved ethical approval in just 18 days in Colombia, and Newrotex obtained authorization in Panama within 15 days. These examples demonstrate the accelerated timelines compared to traditional markets.
How does patient retention in Brazil compare to global averages?
Brazil's patient retention rates are notably 10-15% higher than global averages, which enhances the effectiveness of clinical study execution.
What is the significance of diverse patient pools in the Balkans for clinical trials?
The Balkans offer a rich diversity of patient demographics, which enhances the credibility and validity of research findings. Engaging with diverse patient groups allows researchers to capture a wider array of treatment responses, leading to more effective healthcare solutions.
Why is demographic diversity important in clinical research?
Demographic diversity is crucial for generating generalizable results in clinical trials. It ensures that the innovations developed are representative and beneficial for all segments of society.