


The article defines theranostic biomarkers in cancer treatment, emphasizing their dual role in diagnosis and treatment response prediction. Notable examples include:
These biomarkers are essential for personalized medicine, guiding targeted therapies and enhancing patient outcomes through tailored treatment strategies based on genetic and molecular profiles. By integrating these biomarkers into clinical practice, healthcare professionals can significantly improve the precision of cancer therapies, ultimately leading to better patient care and more effective treatment plans.
In the rapidly evolving landscape of cancer treatment, theranostic biomarkers emerge as pivotal tools that bridge the gap between diagnosis and personalized therapy. These biomarkers not only provide critical insights into tumor characteristics but also guide treatment decisions, ultimately enhancing patient outcomes across various cancer types.
However, with numerous biomarkers available, how can clinicians effectively navigate this complex terrain to optimize treatment strategies?
This article delves into ten key theranostic biomarkers, exploring their definitions, significance, and the latest advancements that are shaping the future of oncology.
HER2/neu is a protein whose overexpression is linked to aggressive forms of breast cancer. Assessing HER2/neu status is crucial for identifying treatment options, including targeted therapies like trastuzumab (Herceptin). This biomarker exemplifies the theranostic biomarkers definition, as it not only assists in diagnosis but also predicts treatment response, establishing it as a cornerstone of personalized medicine in oncology.
Research indicates that patients with HER2-positive breast tumors experience significant benefits from targeted treatments, leading to improved outcomes and survival rates. The 5-year relative survival rate for all stages of breast tumors stands at 91%, while localized breast conditions boast an impressive survival rate of 99%. Moreover, 63.8% of female breast tumors are diagnosed at the local stage, underscoring the importance of early testing and intervention.
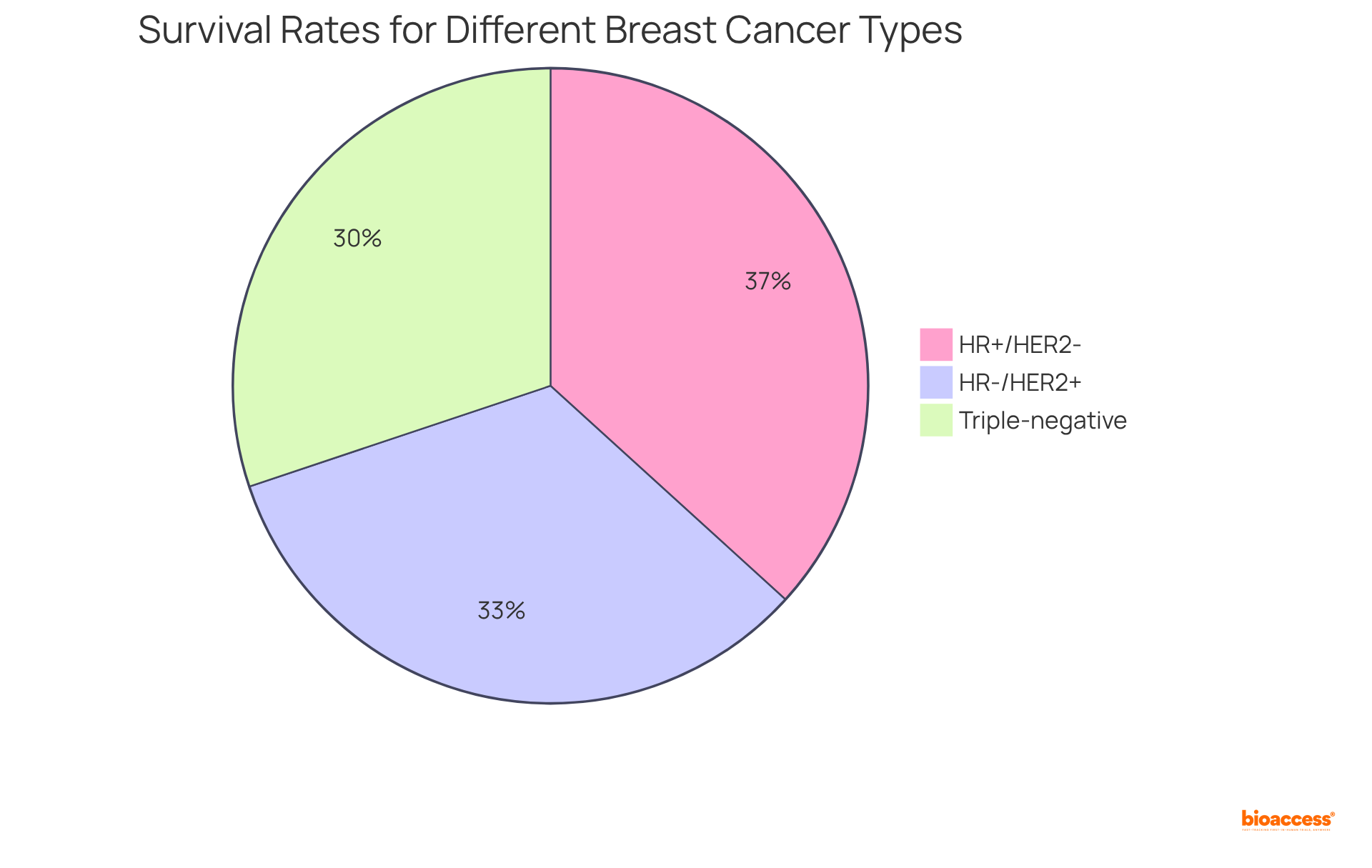
With an estimated 316,950 new cases of female breast disease expected in 2025, the demand for effective testing and management strategies is paramount. According to the National Cancer Institute, survival rates for various breast cancer types are as follows:
Furthermore, the case study titled 'Understanding HER2+ Status and Survival' emphasizes the significant influence of HER2 status on survival rates, reinforcing the critical nature of HER2/neu testing in optimizing treatment strategies.
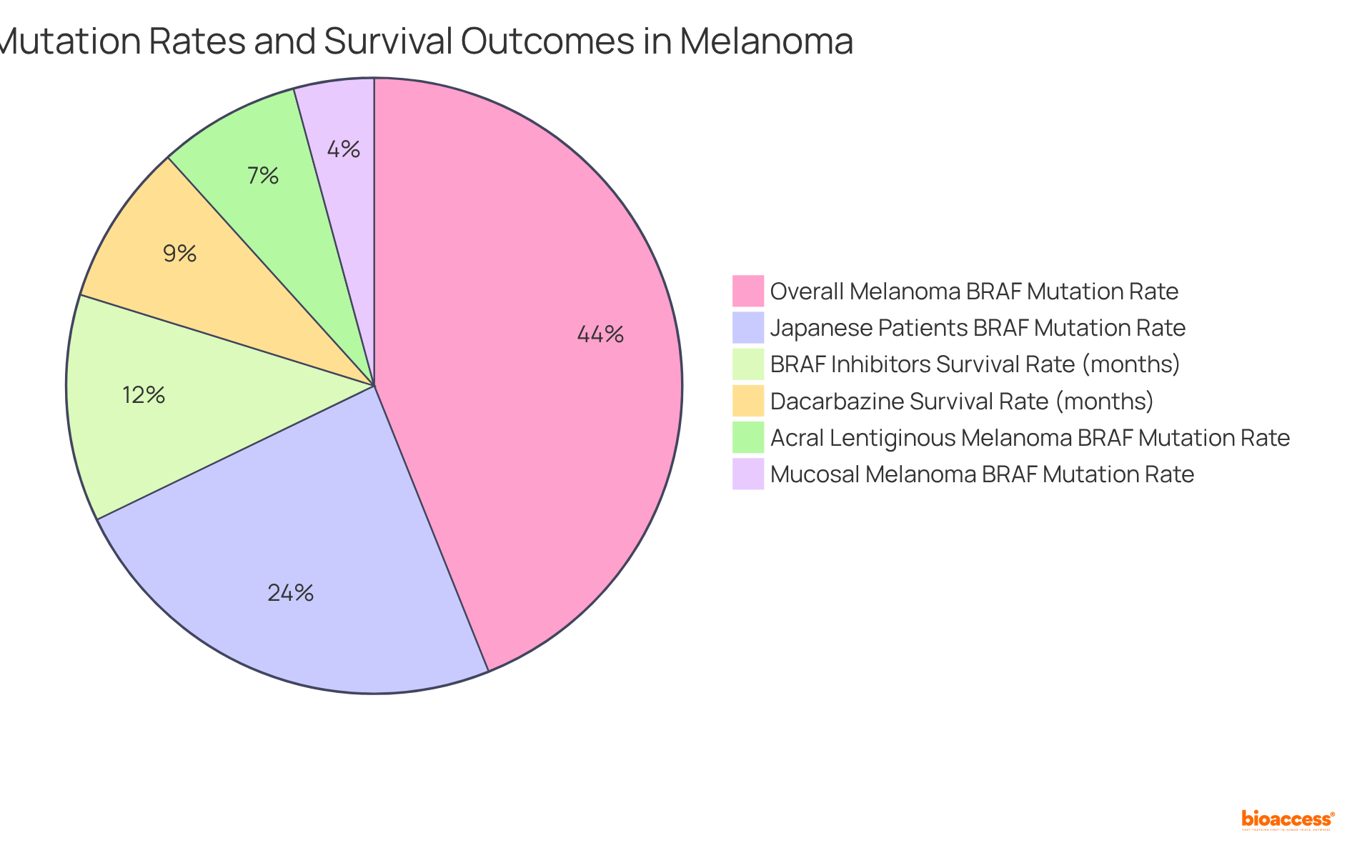
BRAF alterations, particularly the V600E variant, are identified in approximately 40-60% of melanoma cases. Notably, the alteration rate is 27.2% among Japanese melanoma patients, with lower frequencies observed at 8.5% in acral lentiginous melanoma and 4.8% in mucosal melanoma.
Testing for BRAF mutations is crucial, as it informs the application of targeted therapies like BRAF inhibitors (e.g., vemurafenib). These therapies have shown the potential to improve progression-free survival in individuals with BRAF-mutant melanoma, with median overall survival (OS) reported at 13.6 months, compared to 9.7 months with dacarbazine.
Continuous monitoring of BRAF status is essential, given that resistance to therapy can develop within approximately 7 months in nearly half of patients, necessitating timely adjustments in treatment strategies to optimize outcomes.
As Yasuhiro Fujisawa from the Department of Dermatology, Ehime University, underscores, comprehending BRAF mutations is critical for enhancing the effectiveness of melanoma management. Clinicians must prioritize regular BRAF testing and adapt treatment plans accordingly to improve patient outcomes.
Prostate-Specific Antigen (PSA) is a protein produced by prostate cells, and elevated levels often indicate the presence of prostate disease. PSA testing serves as a cornerstone for early detection and ongoing monitoring of disease progression. While increased PSA levels are not exclusively indicative of malignancy, they are crucial in guiding treatment decisions, including the need for biopsy or the initiation of therapy.
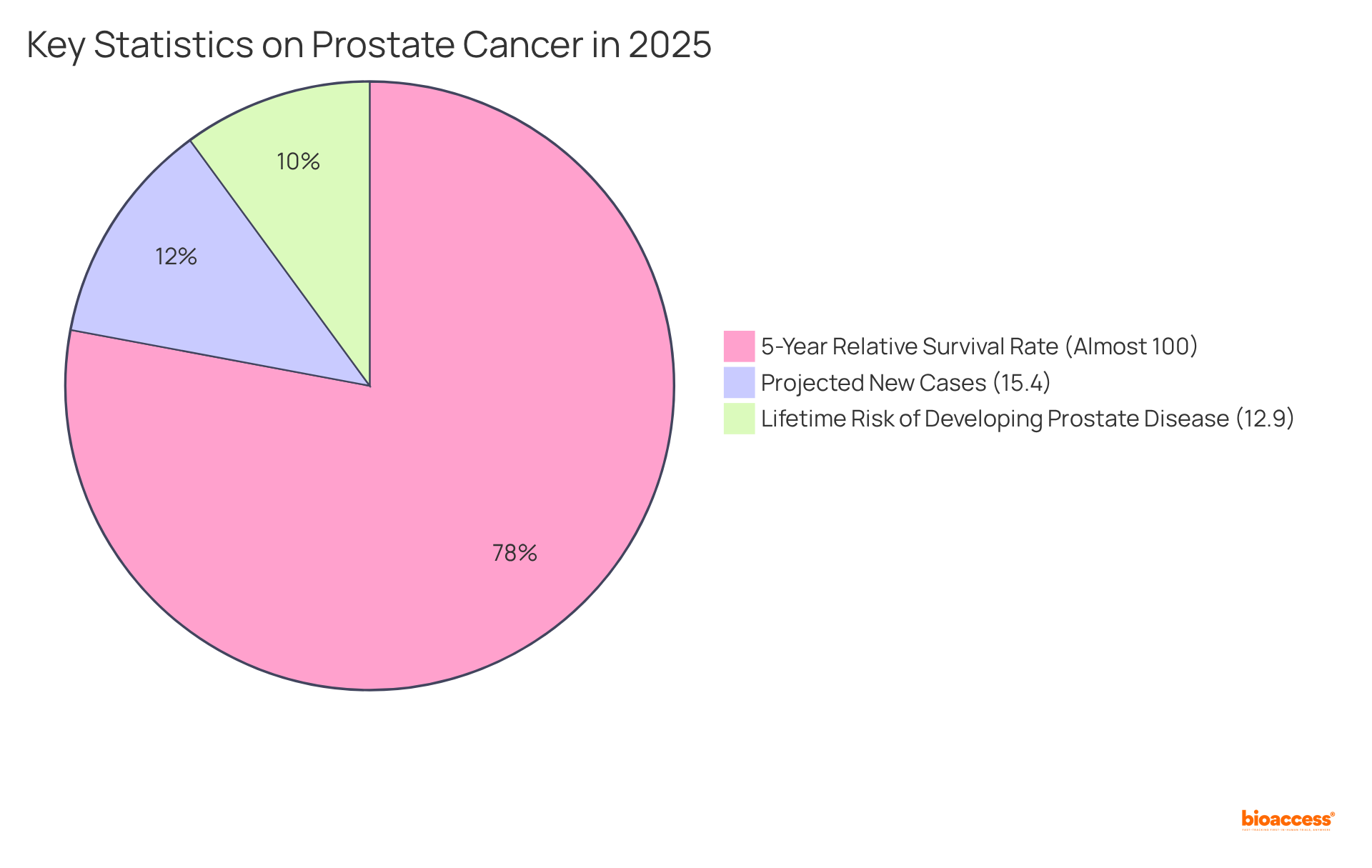
Recent advancements, such as the Prostate Health Index (PHI), have significantly enhanced diagnostic accuracy, facilitating better patient stratification and tailored interventions. This is particularly significant given that prostate malignancy is projected to account for approximately 15.4% of all new tumor cases in the U.S. in 2025, with an estimated 313,780 new diagnoses.
Furthermore, the 5-year relative survival rate for localized prostate disease is nearly 100%, underscoring the effectiveness of early detection. The lifetime risk of developing prostate disease stands at about 12.9%, emphasizing the prevalence of this condition.
Integrating PSA testing into clinical practice not only aids in early detection but also enhances the overall management of prostate conditions, ultimately leading to improved patient outcomes. It is also crucial to consider demographic disparities, as non-Hispanic Black men experience higher mortality rates from prostate disease, highlighting the need for targeted screening and intervention strategies.
KRAS alterations are identified in approximately 40-45% of colorectal tumors and play a significant role in resistance to anti-EGFR treatments. Testing for KRAS alterations is essential for guiding therapeutic approaches, as individuals with KRAS wild-type tumors are substantially more likely to respond positively to treatments such as cetuximab and panitumumab. Conversely, the presence of KRAS alterations necessitates alternative therapeutic strategies, underscoring the importance of personalized medicine in managing colorectal disease.
Notably, recent studies indicate that the most frequently observed KRAS alterations, including G12D, G12V, and G13D, account for about 80% of all identified changes. Additionally, approximately 19% of colorectal cancer patients with confirmed RAS alterations did not exhibit RAS changes in their plasma during treatment adjustments, highlighting the complexity of KRAS testing.
Furthermore, a gender disparity exists, with females exhibiting a higher prevalence of KRAS alterations (48.8%) compared to males (42.6%). The incidence of KRAS alterations varies by region, with reported rates in Turkey ranging from 39.6% to 47.5%, emphasizing the necessity for localized testing.
The overall response rate for KRAS inhibitors in colorectal tumors is around 10%, and mutations in codon 12 are more common than those in codon 13 among patients, which is crucial for understanding the specific types of KRAS mutations that are most prevalent and their implications for treatment.

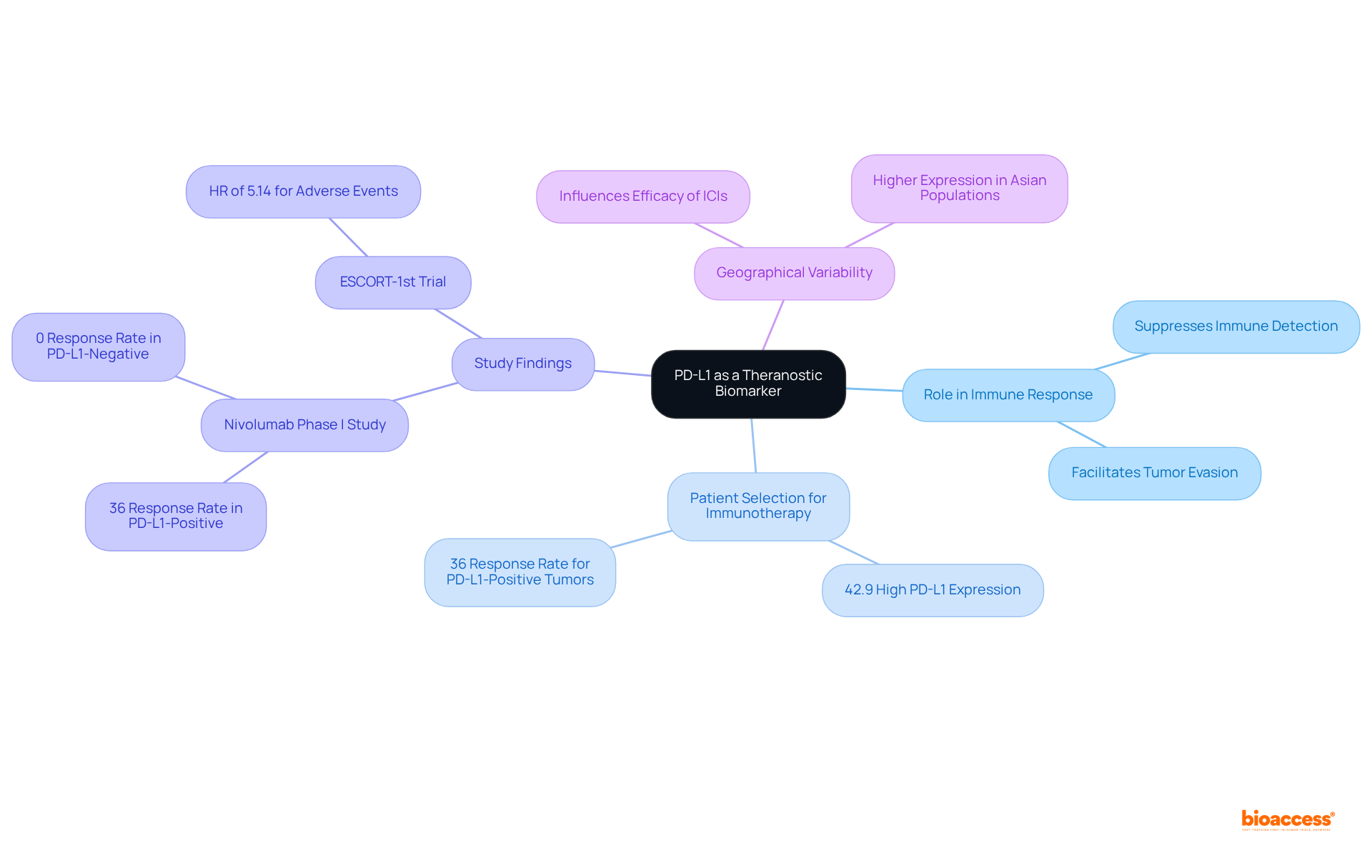
Programmed Death-Ligand 1 (PD-L1) is a pivotal protein that, when overexpressed on tumor cells, can suppress immune responses, allowing malignancies to evade detection. Assessing PD-L1 expression is critical for selecting individuals for immunotherapy, particularly with checkpoint inhibitors such as pembrolizumab and nivolumab. Recent studies indicate that approximately 42.9% of individuals with advanced esophageal malignancy exhibit high PD-L1 expression, defined as a Combined Positive Score (CPS) of 10 or above. This elevated expression is frequently associated with enhanced therapeutic responses, underscoring PD-L1's significance as a vital biomarker in the evolving landscape of cancer treatment.
In a nivolumab phase I study, individuals with PD-L1-positive tumors experienced a 36% response rate, whereas those with PD-L1-negative tumors demonstrated a 0% overall response rate (ORR), further highlighting the predictive value of PD-L1 testing in immunotherapy selection. Furthermore, the effectiveness of PD-L1 inhibitors in real-world settings has been demonstrated, with individuals showing notably improved overall survival (OS) and progression-free survival (PFS) rates when treated with PD-1/PD-L1 inhibitors compared to those lacking such expression.
The ESCORT-1st trial reported a hazard ratio (HR) of 5.14 for overall adverse event rates with combination therapy, emphasizing the necessity of considering the safety profile of PD-L1 inhibitors. Additionally, geographical variability in PD-L1 expression may influence the efficacy of immune checkpoint inhibitors (ICIs) in tumor management. As the understanding of PD-L1's predictive value evolves, its integration into clinical practice is becoming increasingly crucial for optimizing treatment strategies and enhancing patient outcomes.
Anaplastic Lymphoma Kinase (ALK) rearrangements occur in a subset of non-small cell lung tumors (NSCLC) and represent actionable targets for treatment. Testing for ALK rearrangements is essential, as it determines eligibility for ALK inhibitors such as crizotinib and alectinib. These targeted therapies have demonstrated significant efficacy in patients with ALK-positive NSCLC, resulting in improved response rates and survival outcomes. Furthermore, continuous monitoring of ALK status is critical due to the potential emergence of resistance mechanisms.
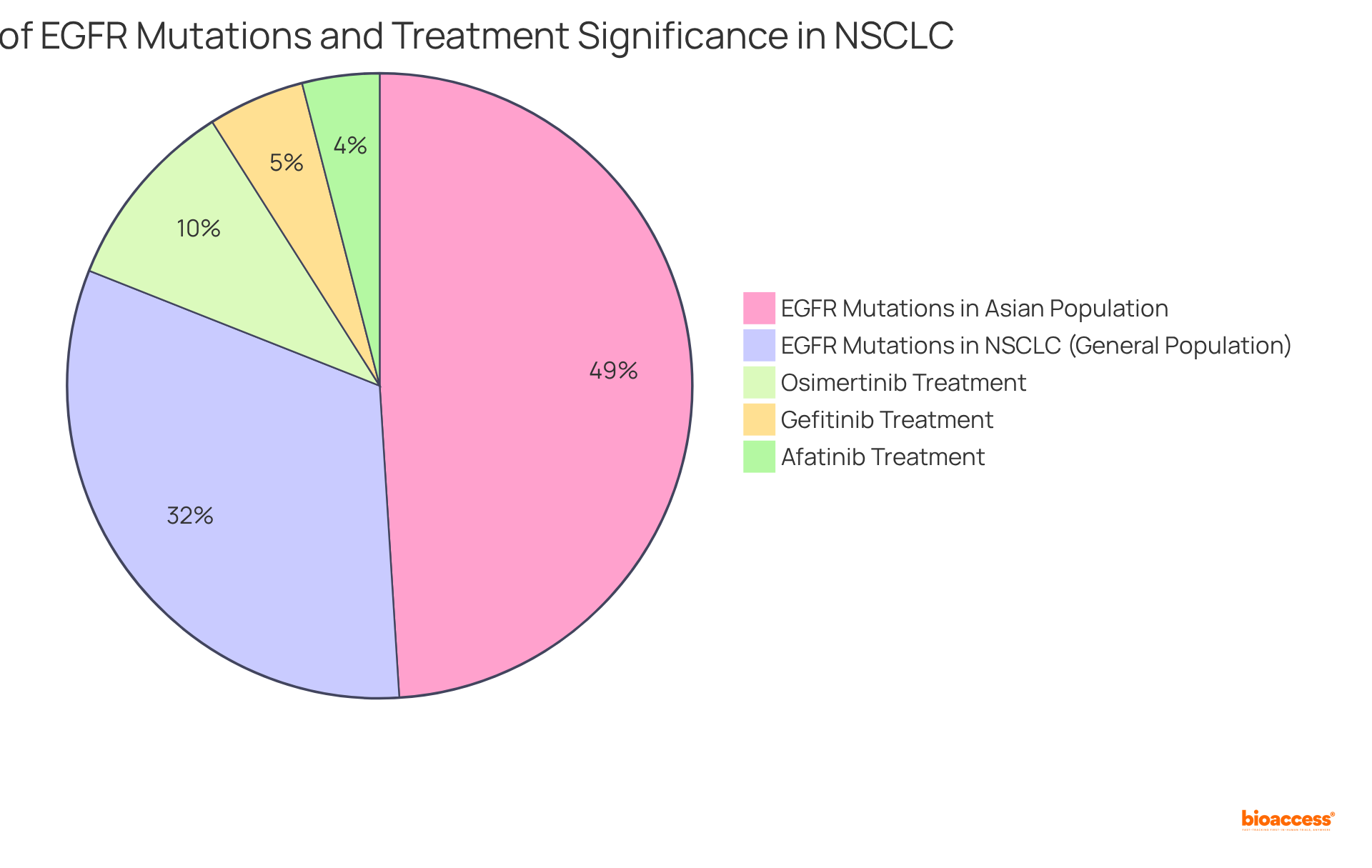
Epidermal Growth Factor Receptor (EGFR) alterations are prevalent in approximately 32% of non-small cell lung cancer (NSCLC) cases, playing a pivotal role in shaping treatment strategies. Assessing these alterations is vital, as it directly impacts the use of EGFR tyrosine kinase inhibitors (TKIs) such as osimertinib, authorized in 2015, which has demonstrated greater effectiveness compared to traditional chemotherapy.
Patients with EGFR alterations—especially those from Asian groups, where alteration rates can reach approximately 49%—typically achieve better outcomes with targeted treatments. This underscores the significance of regular EGFR testing, which is crucial not only for optimizing initial care plans but also for managing potential resistance, allowing for timely adjustments to therapeutic strategies.
Recent advancements in EGFR mutation testing have further enhanced treatment precision, ensuring individuals receive the most effective therapies tailored to their specific genetic profiles. Notably, gefitinib and afatinib were approved in 2015 and 2013, respectively, marking significant milestones in the development of targeted therapies for NSCLC.
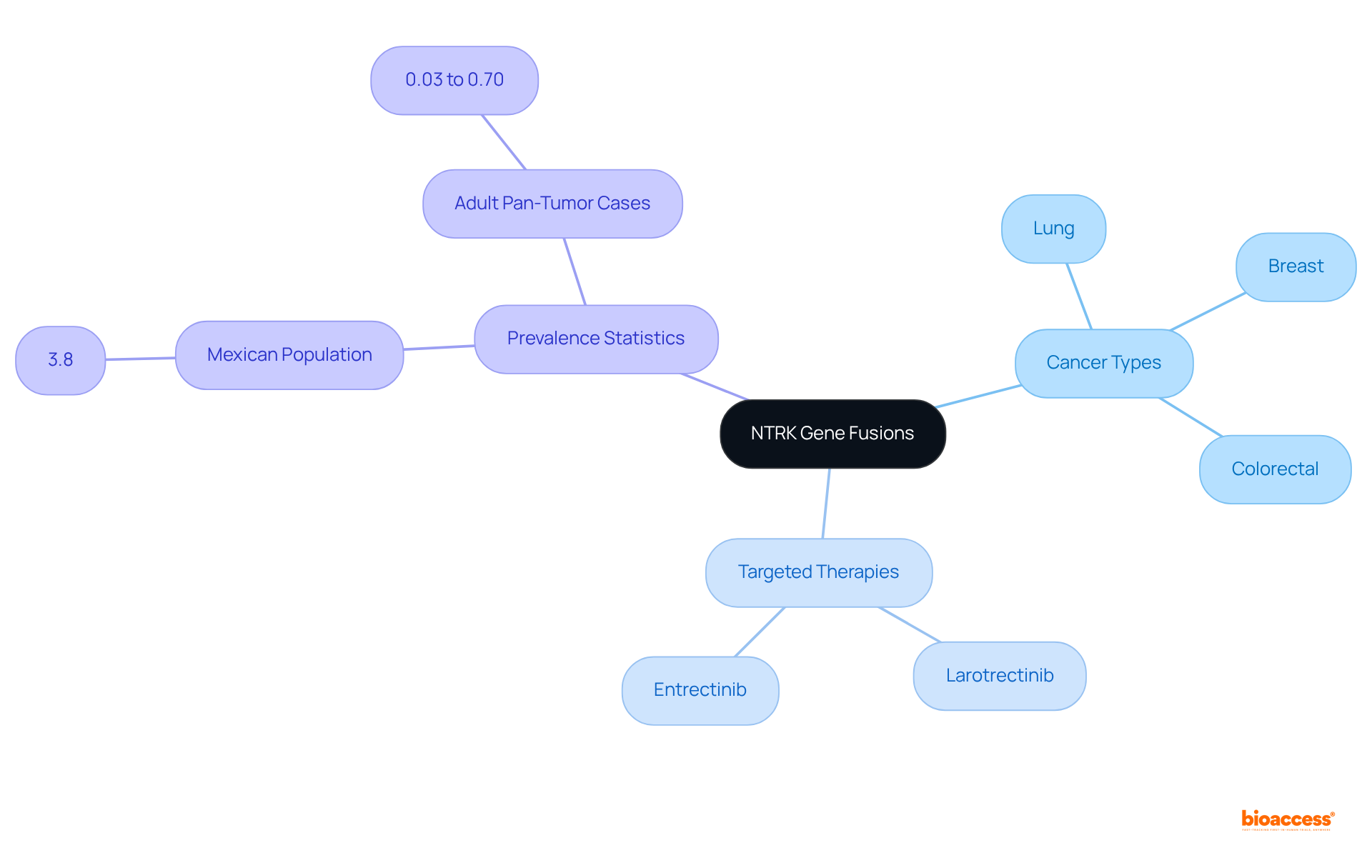
NTRK gene fusions are increasingly recognized as pivotal biomarkers across various cancers, including lung, breast, and colorectal types. Testing for these fusions is essential, as it enables the administration of targeted therapies such as larotrectinib and entrectinib, which have demonstrated significant effectiveness in individuals with NTRK fusion-positive tumors. Notably, larotrectinib is FDA-approved for both adult and pediatric patients with solid tumors that harbor an NTRK gene fusion.
Recent studies indicate that approximately 3.8% of the Mexican population analyzed displayed NTRK gene fusions, while estimates for adult pan-tumor cases vary from 0.03% to 0.70%. This underscores the critical need for molecular profiling in personalized oncology. Such targeted approaches not only enhance treatment outcomes but also emphasize the importance of integrating NTRK testing into clinical practice.
The practical application of these therapies has yielded encouraging results, as evidenced by a retrospective chart review identifying a cohort of 512 patients with NTRK fusion-positive solid tumors. This reinforces the necessity of precise genomic testing in optimizing treatment strategies. However, challenges persist in the integration of these treatments into healthcare systems, highlighting the need for accurate prevalence estimates to inform health economic analyses.
Microsatellite instability (MSI) is a crucial indicator of genetic hypermutability linked to various tumors, particularly colorectal and endometrial cancers. Understanding MSI status is vital, as it identifies patients who may benefit from immunotherapy, such as pembrolizumab, which is specifically approved for tumors classified as MSI-high.
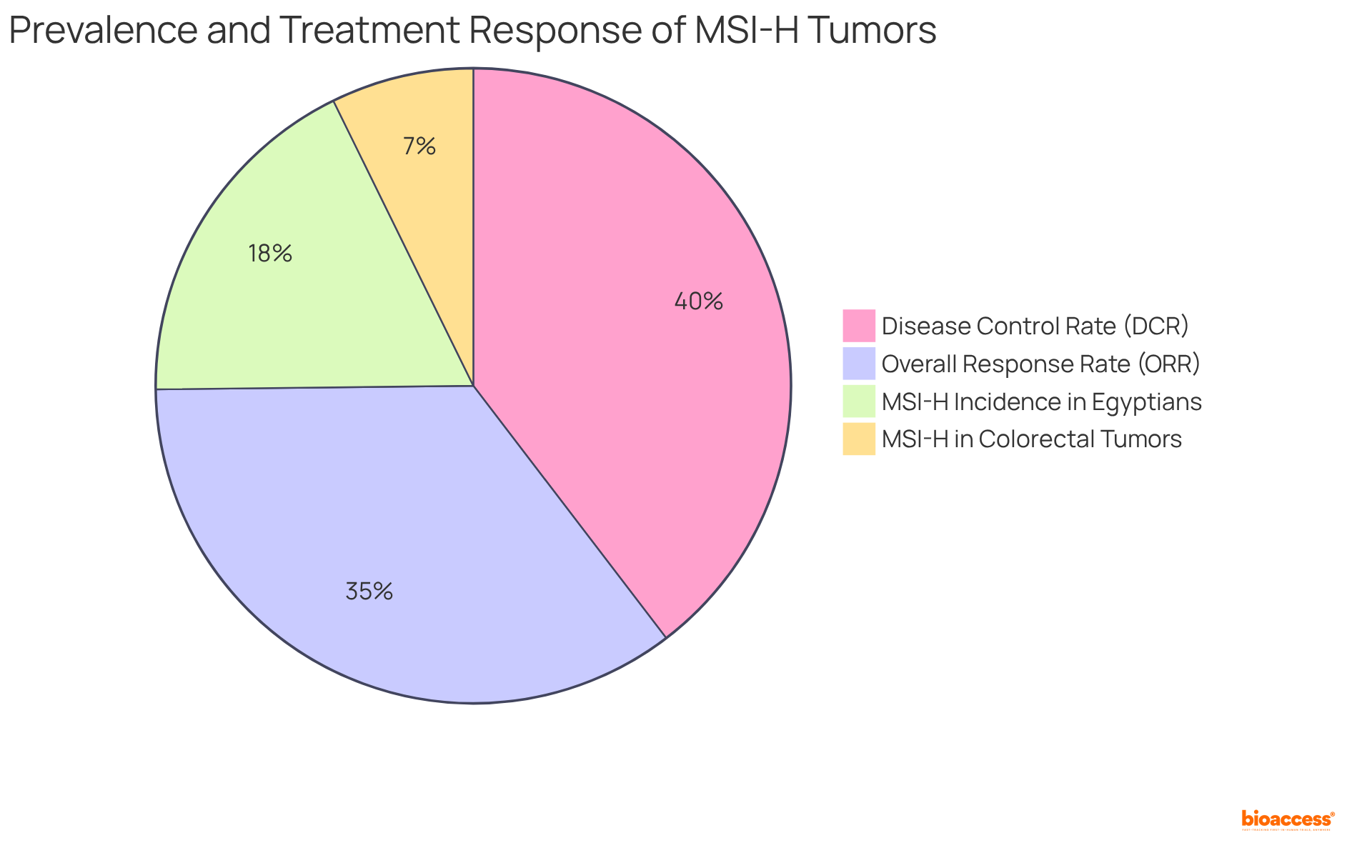
Approximately 15% of colorectal tumors present with MSI-H or deficient mismatch repair (dMMR), with the incidence of MSI-H in colorectal cancer being notably highest among Egyptians, reaching 37%. This underscores the significance of MSI as a predictive biomarker in treatment decision-making. The presence of MSI indicates a deficiency in the DNA mismatch repair system, which is essential for guiding therapeutic strategies and enhancing patient outcomes.
Recent studies have shown that individuals with MSI-H tumors frequently enjoy better prognoses and are more likely to respond favorably to immune checkpoint inhibitors. For instance, the KeyNote177 trial demonstrated that pembrolizumab significantly improved progression-free survival compared to traditional chemotherapy in patients with MSI-H colorectal disease.
Furthermore, real-world data suggest that the overall response rate (ORR) for immunotherapy in MSI-H tumors can soar to 72.7%, with a disease control rate (DCR) of 81.8%. As noted by Ashish Nepal, 'Within our small, isolated sample, the overall response rate (ORR) was 72.7%, and the disease control rate (DCR) was 81.8% with 6 CR and 2 PR.' This illustrates the transformative role of MSI testing in treatment decisions, facilitating personalized approaches that enhance therapeutic effectiveness.
Additionally, the increasing prevalence of MSI/MMR testing reflects a growing awareness and clinical suspicion of MSI-H/dMMR across various diseases, further emphasizing the critical role of MSI testing in contemporary clinical practices. It is also imperative to screen for Lynch syndrome in patients with positive MSI-H or dMMR results, as this represents a fundamental aspect of patient management.
Circulating tumor DNA (ctDNA) represents a critical advancement in cancer monitoring, consisting of DNA fragments released by tumors into the bloodstream. This non-invasive method facilitates the detection of mutations, evaluation of treatment responses, and monitoring for recurrences, establishing it as an invaluable tool in clinical practice.
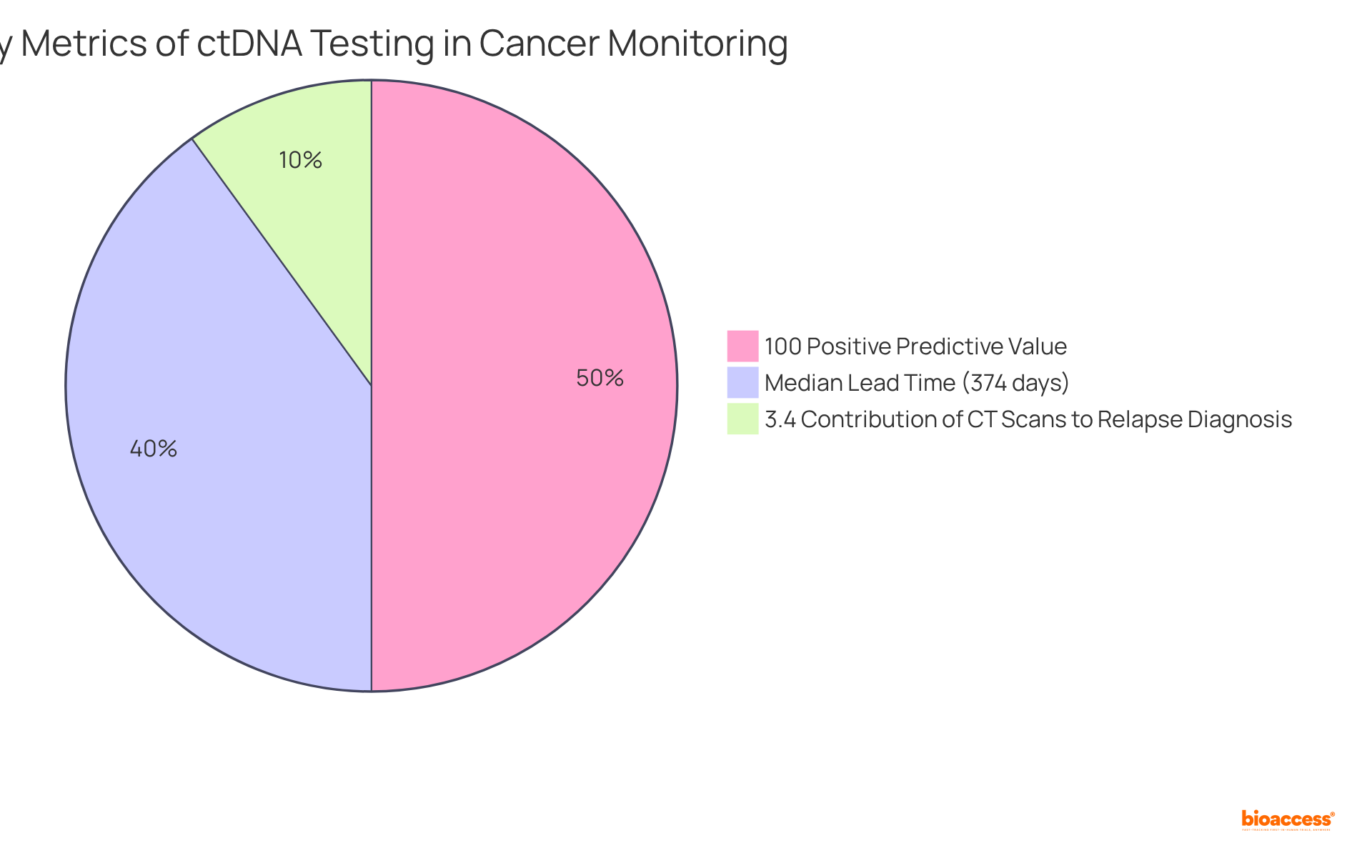
Recent studies underscore ctDNA's potential as a biomarker that not only provides real-time insights into tumor dynamics but also informs therapy adjustments, thereby enhancing management. For instance, ctDNA monitoring has demonstrated a 100% positive predictive value (PPV) for predicting recurrence, alongside high sensitivity and specificity in detecting clinical relapses.
Studies indicate a median lead time of 374 days (ranging from 13 to 1010 days) from ctDNA detection to clinical recurrence, enabling prompt interventions that can significantly improve outcomes for individuals. Furthermore, the integration of ctDNA testing into standard monitoring has shown promise in reducing unnecessary imaging procedures, with only 3.4% of CT scans contributing to a relapse diagnosis. This optimization of care ensures efficient observation of therapeutic responses.
Overall, ctDNA testing is becoming increasingly essential in the realm of cancer management, offering a personalized approach to treatment that aligns with the evolving needs of patients.
The exploration of theranostic biomarkers in cancer treatment reveals their pivotal role in personalizing and optimizing therapeutic strategies. By integrating diagnostic and therapeutic capabilities, these biomarkers not only enhance treatment efficacy but also significantly improve patient outcomes across various cancer types. The insights provided into biomarkers such as HER2/neu, BRAF mutations, PSA, KRAS, PD-L1, ALK rearrangements, EGFR mutations, NTRK fusions, MSI, and ctDNA collectively underscore the transformative potential of precision medicine in oncology.
Key arguments presented throughout the article highlight the necessity of testing and monitoring these biomarkers to inform treatment decisions. For instance:
As the landscape of cancer treatment continues to evolve, the integration of theranostic biomarkers into clinical practice is not just beneficial but essential. Emphasizing the importance of early detection and tailored therapies can lead to significantly improved survival rates and quality of life for patients. Healthcare professionals and researchers must remain committed to advancing the understanding and application of these biomarkers, ensuring that personalized medicine becomes a standard practice in oncology.
What is HER2/neu and its significance in breast cancer?
HER2/neu is a protein whose overexpression is linked to aggressive forms of breast cancer. Assessing HER2/neu status is crucial for identifying treatment options, including targeted therapies like trastuzumab (Herceptin), making it a key theranostic biomarker in personalized medicine.
How does HER2/neu status affect breast cancer treatment outcomes?
Patients with HER2-positive breast tumors benefit significantly from targeted treatments, leading to improved outcomes and survival rates. The 5-year relative survival rate for all stages of breast tumors is 91%, while localized breast conditions have a survival rate of 99%.
What are the survival rates for different breast cancer types based on HER2/neu status?
According to the National Cancer Institute, survival rates are as follows: HR+/HER2- at 95.1%, HR-/HER2+ at 85.7%, and triple-negative breast cancers have the lowest survival rates.
Why is BRAF mutation testing important in melanoma management?
BRAF mutations, particularly the V600E variant, are present in 40-60% of melanoma cases, and testing for these mutations is crucial to apply targeted therapies like BRAF inhibitors, which can improve progression-free survival.
What are the expected survival outcomes for patients with BRAF-mutant melanoma?
The median overall survival for patients with BRAF-mutant melanoma treated with BRAF inhibitors is reported at 13.6 months, compared to 9.7 months for those treated with dacarbazine.
How often should BRAF status be monitored in melanoma patients?
Continuous monitoring of BRAF status is essential, as resistance to therapy can develop within approximately 7 months in nearly half of the patients, necessitating timely adjustments in treatment strategies.
What role does Prostate-Specific Antigen (PSA) play in prostate cancer?
PSA is a protein produced by prostate cells, and elevated levels often indicate prostate disease. PSA testing is vital for early detection and monitoring disease progression, guiding treatment decisions.
What advancements have improved PSA testing accuracy?
The Prostate Health Index (PHI) has significantly enhanced diagnostic accuracy, allowing for better patient stratification and tailored interventions in prostate cancer management.
What is the projected incidence of prostate cancer in the U.S. for 2025?
Prostate malignancy is projected to account for approximately 15.4% of all new tumor cases in the U.S. in 2025, with an estimated 313,780 new diagnoses.
What is the survival rate for localized prostate disease?
The 5-year relative survival rate for localized prostate disease is nearly 100%, highlighting the effectiveness of early detection.
What demographic disparities exist in prostate cancer mortality rates?
Non-Hispanic Black men experience higher mortality rates from prostate disease, emphasizing the need for targeted screening and intervention strategies.