


The article examines the pivotal role of Contract Research Organizations (CROs) in enhancing research efficiency within clinical trials. By implementing various strategies—such as streamlined regulatory compliance, diverse patient recruitment, and the integration of advanced technology—CROs significantly improve operational timelines and reduce costs. This is particularly evident in their focus on rapid ethical approvals and effective project management, which collectively accelerate the path to commercialization. Moreover, these efforts enhance patient access to innovative therapies, underscoring the importance of CROs in the evolving landscape of clinical research.
In the fast-paced world of medical research, the efficiency of clinical trials is a critical factor determining whether innovative therapies reach patients. Contract Research Organizations (CROs) are rising to the challenge, offering a variety of strategies that enhance research efficiency and streamline processes. Yet, how do these organizations adeptly navigate the complexities of ethical approvals, regulatory compliance, and patient recruitment to ensure successful outcomes? This article delves into ten pivotal ways in which CROs are transforming clinical trials, ultimately accelerating the journey from research to real-world application.
The company leverages its profound understanding of regulatory frameworks across Latin America, the Balkans, and Australia to secure ethical approvals in just 4-6 weeks. This rapid transformation is essential for Medtech, Biopharma, and Radiopharma pioneers striving to expedite their research phases. By streamlining the approval process, bioaccess® empowers clients to initiate cross clinical trials more swiftly, ultimately accelerating the path to commercialization and enhancing patient access to innovative therapies. Our comprehensive CRO clinical trial services in Colombia encompass:
With our global network of fast-track clinical trial sites, we can reduce FDA/EU submission timelines by 6-12 months, providing a competitive edge in patient recruitment and site activation. This innovative approach not only enhances research efficiency but also stimulates economic growth and improves healthcare outcomes in the regions we serve.
The company places a strong emphasis on regulatory compliance by continuously updating its knowledge of the latest guidelines and protocols. This proactive strategy guarantees that all medical studies adhere to both local and international regulations, greatly reducing the risk of compliance-related delays or setbacks.
Significantly, this product provides a faster regulatory approval procedure, securing approvals in merely 6-8 weeks versus the usual 6-12 months in the US/EU. This efficiency is enhanced by the capability to enroll treatment-naive cardiology or neurology groups 50% quicker than Western locations, which is essential for addressing regulatory hurdles in early-phase clinical studies.
By adopting comprehensive compliance strategies, bioaccess® not only protects the integrity of its research but also bolsters the credibility of its findings. This credibility is crucial for attracting investors and stakeholders, as regulatory compliance is directly connected to the reliability and validity of research outcomes.
Research indicates that pharmaceutical firms utilizing strong data management systems can lower their clinical study expenses by an average of 25%, while those that emphasize compliance enhance their chances of regulatory approval by 23%. Additionally, effective compliance practices enhance patient safety and ensure that studies are conducted ethically, which is essential for maintaining public trust and advancing medical knowledge.
To ensure regulatory compliance, the organization employs best practices such as:
These measures not only facilitate adherence to good clinical practice (GCP) guidelines but also promote transparency and accountability throughout the research process.
The company strategically leverages its operations across Latin America, the Balkans, and Australia to tap into a rich diversity of patient demographics for cros clinical trials, significantly enhancing recruitment strategies. This diversity not only accelerates enrollment but also ensures that study populations are more representative of the broader community.
By engaging local networks and implementing targeted community outreach initiatives, the organization effectively connects with potential participants. This method is essential, as almost 80% of research studies do not complete on schedule, frequently due to recruitment difficulties.
Additionally, with average enrollment durations differing by area, the system's capacity to manage these challenges facilitates prompt completion and the attainment of required sample sizes. Successful patient recruitment strategies, such as fostering relationships with local advocacy groups and utilizing digital platforms for outreach, have proven effective in increasing participation rates.
By integrating these strategies into their operational framework, the organization not only improves recruitment efficiency but also supports the overall success of cros clinical trials.
This company provides affordable clinical study solutions that empower clients to optimize their funding effectively. By leveraging local resources and enhancing operational efficiencies, bioaccess® guarantees high-quality services at competitive prices. This strategic approach not only alleviates the financial burden on clients but also enables them to reallocate resources to other critical areas of their development initiatives.
Considering that research studies can cost between USD 266 million and USD 802 million to bring a new drug to market, effective budget management becomes essential. Collaborating with a partner allows clients to benefit from a streamlined process that improves resource allocation and accelerates timelines, ultimately leading to more impactful and significant outcomes.
At bioaccess®, a team of seasoned experts with extensive research backgrounds spearheads the development of robust study designs tailored to specific research objectives. This expertise not only enhances the execution of medical studies but also ensures these studies are conducted efficiently, yielding reliable results.
Organizations that leverage experienced teams often report a significant improvement in study outcomes, with research indicating that collaboration with a research partner positively influences success rates. Furthermore, the integration of innovative strategies, such as adaptive designs and AI-driven analytics, facilitates real-time adjustments based on interim data, thereby enhancing overall study efficiency.
Industry leaders emphasize that effective collaboration and communication are vital, with 72% of sponsors asserting that improved training would greatly enhance site performance. By prioritizing these best practices, bioaccess® illustrates how skilled teams can elevate the quality and efficiency of research studies, ultimately leading to faster and more successful outcomes.
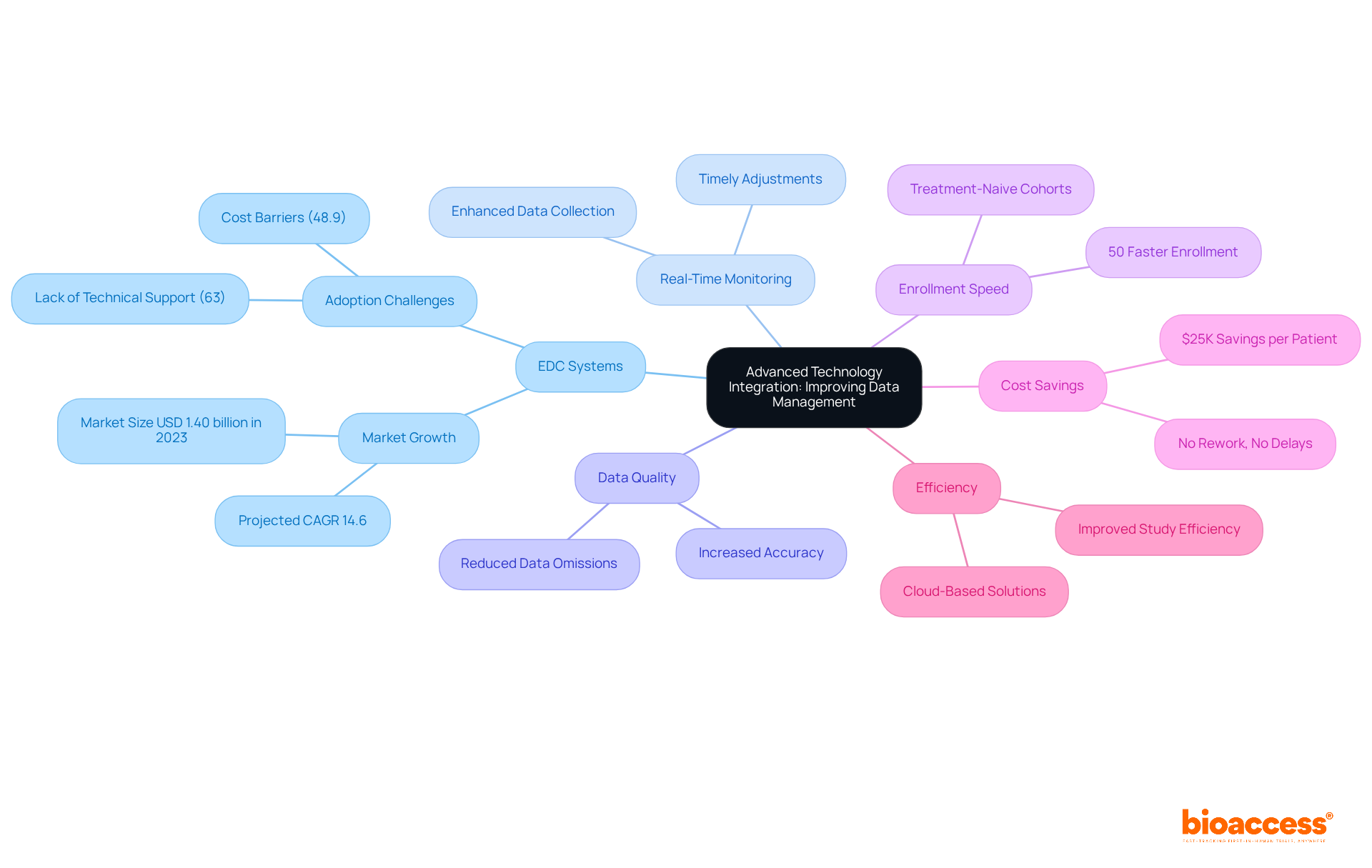
The system employs advanced technology to significantly enhance data management and analysis within cros clinical trials. By implementing electronic data capture (EDC) systems and real-time monitoring tools, the company guarantees precise and efficient data collection. This technological advancement notably improves data quality and accelerates the reporting process, which facilitates quicker decision-making and timely adjustments during experiments.
Importantly, bioaccess® can enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, yielding substantial savings of $25K per patient with FDA-ready data—no rework, no delays.
Furthermore, studies indicate that EDC systems can reduce data omissions and enhance overall study efficiency in cros clinical trials, making them indispensable for modern medical investigations. Additionally, cloud-based EDC solutions have demonstrated their ability to simplify study design and data gathering, ultimately leading to more effective and efficient medical research.
The company employs flexible project management strategies that enable swift adaptations to the evolving needs of research. This agility is particularly crucial in cros clinical trials, where unexpected obstacles—such as regulatory hurdles, competition, and recruitment issues—can arise. By maintaining open channels of communication with clients and stakeholders, the company can promptly modify timelines, resources, and strategies, ensuring that experiments progress smoothly. Furthermore, the company offers comprehensive services, including:
These services further enhance the efficient execution of cros clinical trials.
The organization cultivates a culture of collaborative innovation by engaging with a diverse array of stakeholders, including investigators, sponsors, and regulatory bodies. This collaborative structure not only enables the sharing of concepts and resources but also significantly enhances study results. By leveraging the combined knowledge of all stakeholders, bioaccess® can devise inventive solutions that effectively tackle the complex challenges inherent in medical studies.
Successful partnerships, such as those seen in the development of Charco Neurotech's wearable device for Parkinson's, exemplify how collaboration can lead to groundbreaking advancements in medical technology. Industry leaders underscore the vital role of stakeholder collaboration; as one remarked, 'The best inquiry you can conduct is to converse with individuals.' This sentiment underscores the importance of open communication and shared knowledge in achieving trial success.
Ultimately, partnerships not only streamline processes but also enrich the research landscape, paving the way for more effective and efficient cross clinical trials.
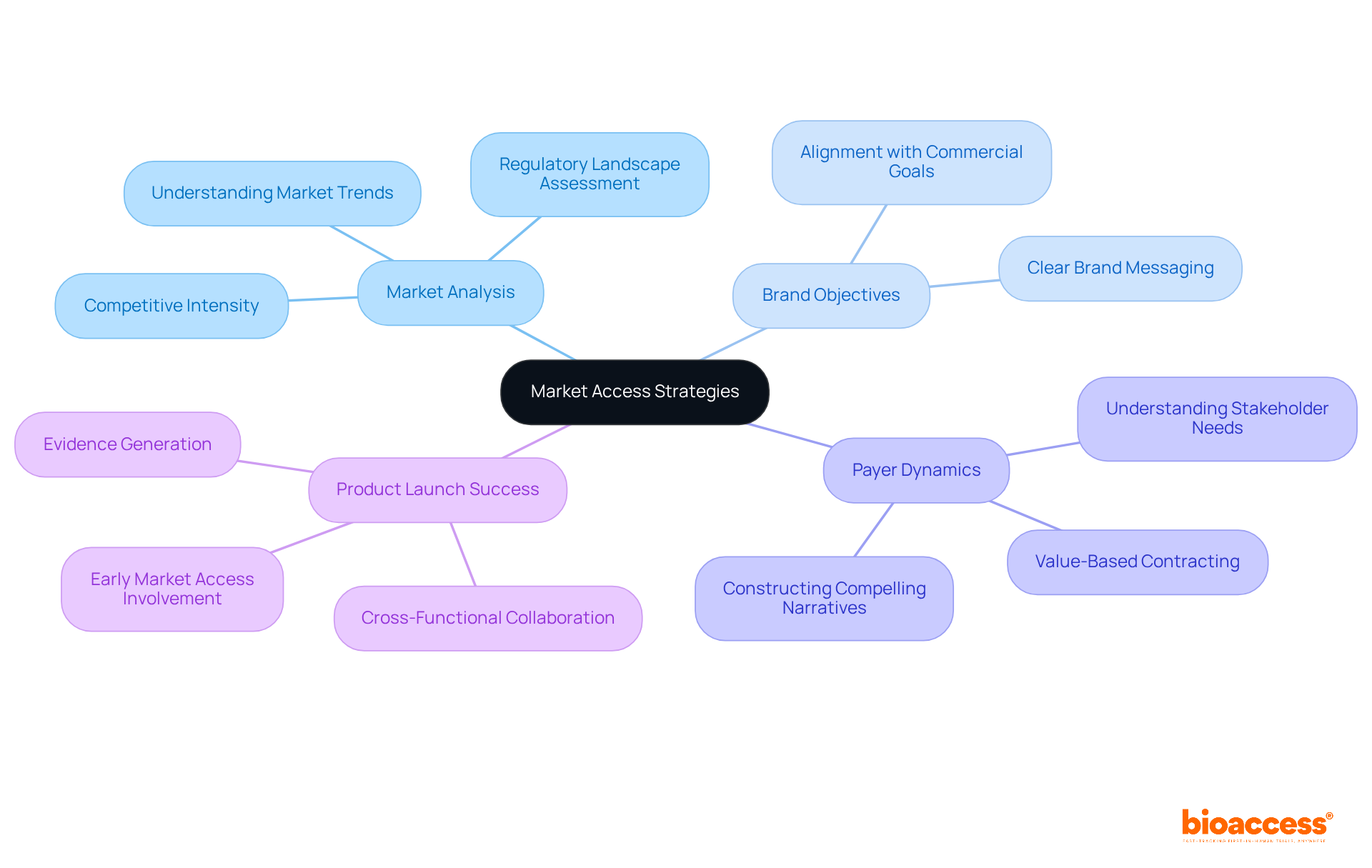
The company provides robust market access strategies that empower clients to successfully launch their products in competitive healthcare environments. By meticulously analyzing market trends and regulatory landscapes, the organization delivers essential insights that enable clients to navigate the commercialization process effectively. This strategic support significantly enhances the likelihood of successful product launches, as research indicates that 57% of drug launch failures arise from limited market access.
Furthermore, a disciplined approach to market access, initiated with clear brand objectives aligned with commercial goals, maximizes return on investment for clients. As noted by a market access professional, understanding payer dynamics is crucial for constructing a compelling narrative that resonates with stakeholders. By leveraging these insights, the company ensures that clients are well-prepared to confront market challenges, ultimately enhancing their product launch success.
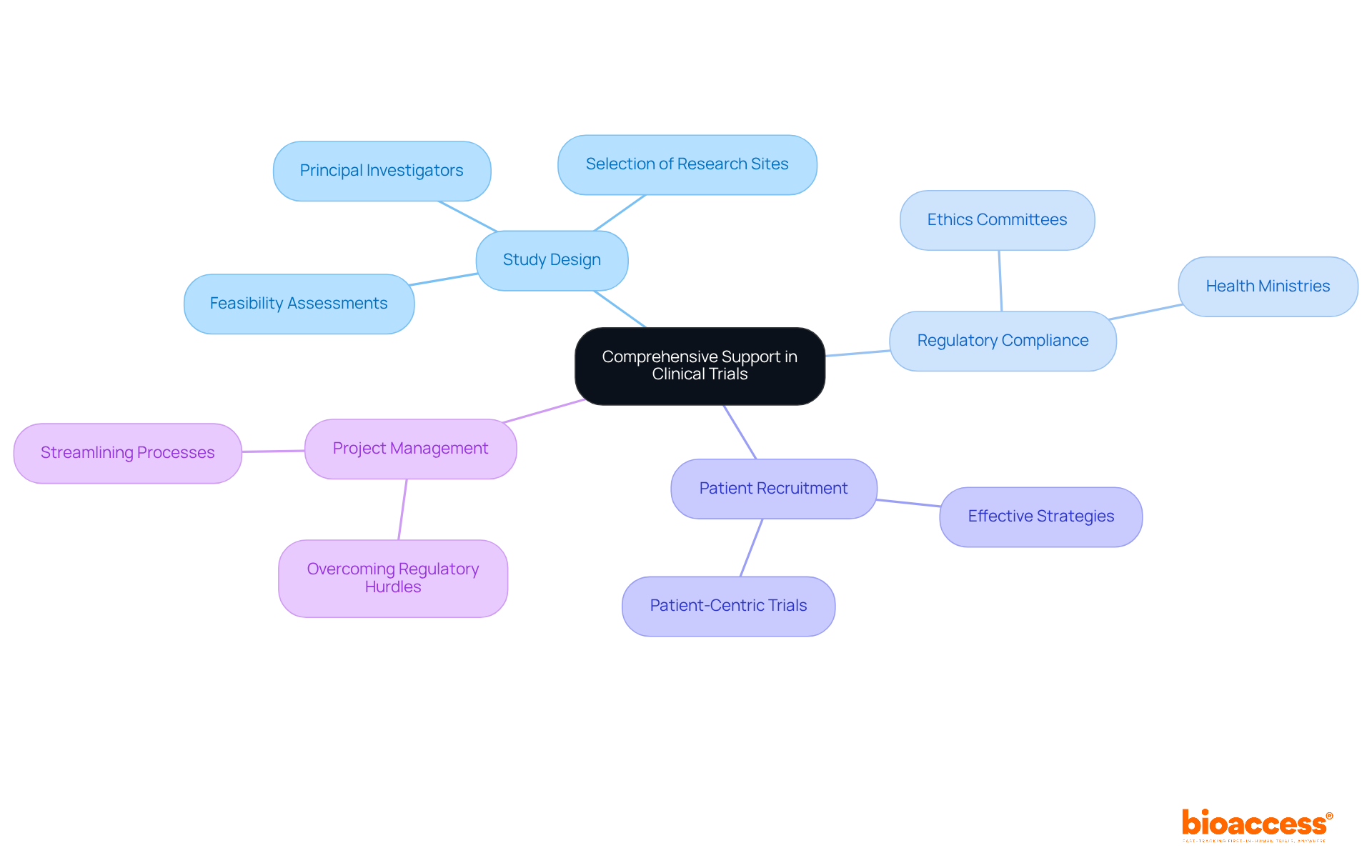
The platform delivers robust support throughout the cros clinical trials process, encompassing everything from initial study design to final reporting. This comprehensive service includes:
All while ensuring regulatory compliance with ethics committees and health ministries. In addition, the company provides effective patient recruitment strategies and project management services, which are crucial for overcoming the common challenges faced by medical device startups, such as regulatory hurdles and recruitment difficulties. By offering a complete suite of services, bioaccess® alleviates the burdens on clients, enabling them to focus on their core competencies while guaranteeing that their cros clinical trials are executed efficiently and effectively.
The insights presented highlight the transformative role that Contract Research Organizations (CROs) play in enhancing the efficiency of clinical trials. By streamlining processes such as ethical approvals, regulatory compliance, and patient recruitment, CROs like bioaccess® significantly reduce timelines and costs while improving the overall quality of research. This strategic approach not only accelerates the path to market for innovative therapies but also ensures that studies are conducted ethically and responsibly.
Key arguments throughout the article emphasize the importance of:
The ability to access diverse patient pools and implement cost-effective solutions further underscores the value that CROs bring to the table, facilitating successful product launches and ultimately improving healthcare outcomes.
In conclusion, the collaboration between CROs and research sponsors is essential for navigating the complexities of clinical trials. By adopting best practices and innovative strategies, stakeholders can enhance research efficiency and drive advancements in medical science. Embracing these insights and solutions not only paves the way for more effective clinical trials but also contributes to a healthier future for patients worldwide.
What is bioaccess and what services does it provide?
bioaccess is a company that accelerates clinical trials by securing ethical approvals in 4-6 weeks across Latin America, the Balkans, and Australia. Its services include study design, feasibility assessments, coordination of regulatory submissions, and efficient preparation and submission of necessary documentation.
How does bioaccess expedite the clinical trial approval process?
bioaccess streamlines the approval process by leveraging its understanding of regulatory frameworks, enabling clients to initiate cross clinical trials more swiftly and thus accelerating the path to commercialization.
What are the benefits of using bioaccess for clinical trials?
Using bioaccess can reduce FDA/EU submission timelines by 6-12 months, enhance patient recruitment, and improve site activation, ultimately leading to faster access to innovative therapies for patients.
How does bioaccess ensure regulatory compliance in clinical trials?
bioaccess ensures regulatory compliance by continuously updating its knowledge of guidelines, employing best practices such as thorough training for trial personnel, conducting regular audits, and using integrated data management systems.
What is the typical timeline for securing regulatory approvals through bioaccess?
bioaccess can secure regulatory approvals in 6-8 weeks, compared to the usual 6-12 months in the US/EU.
How does bioaccess enhance patient recruitment for clinical trials?
bioaccess enhances patient recruitment by leveraging its operations across diverse regions, engaging local networks, implementing targeted outreach initiatives, and fostering relationships with local advocacy groups.
Why is diversity in patient demographics important for clinical trials?
Diversity in patient demographics ensures that study populations are more representative of the broader community, which can enhance the validity of research outcomes and improve recruitment efficiency.
What are the consequences of poor recruitment in clinical trials?
Poor recruitment can lead to delays, with almost 80% of research studies not completing on schedule due to recruitment difficulties.
How do compliance practices impact the success of clinical trials?
Strong compliance practices enhance the chances of regulatory approval, improve patient safety, and maintain public trust, which are all crucial for the integrity and credibility of research findings.