


Post-Authorization Safety Studies (PASS) are crucial for safeguarding public health by monitoring the long-term effects of medications once they reach the market. In Serbia, these studies transcend mere regulatory obligations; they are vital for enhancing patient safety and ensuring that the benefits of drugs significantly outweigh their risks.
As the landscape shifts with new regulations set to take effect in 2025, sponsors and researchers face the challenge of navigating compliance complexities while preserving the integrity of their studies. Understanding the key requirements for conducting PASS in Serbia is essential for anyone involved in pharmacovigilance and clinical research.
Post-Authorization Safety Studies (PASS) are critical for pharmacovigilance and are carried out to fulfill the post-authorization safety study requirements in Serbia after a drug has received marketing authorization. These investigations are essential for gathering additional data on safety and assessing the long-term effects of medications in real-world settings. The regulatory framework governing the post-authorization safety study requirements in Serbia is aligned with European Union standards, ensuring that research is meticulously designed to monitor adverse effects and evaluate the benefit-risk profile of approved medications.
This proactive approach not only enhances patient well-being but also supports regulatory decision-making processes. As of January 2025, marketing authorization holders will be mandated to utilize the IRIS platform for managing PASS, which will streamline data collection and reporting processes. This shift underscores the commitment to improving safety monitoring and reinforces the importance of collaboration in clinical research.
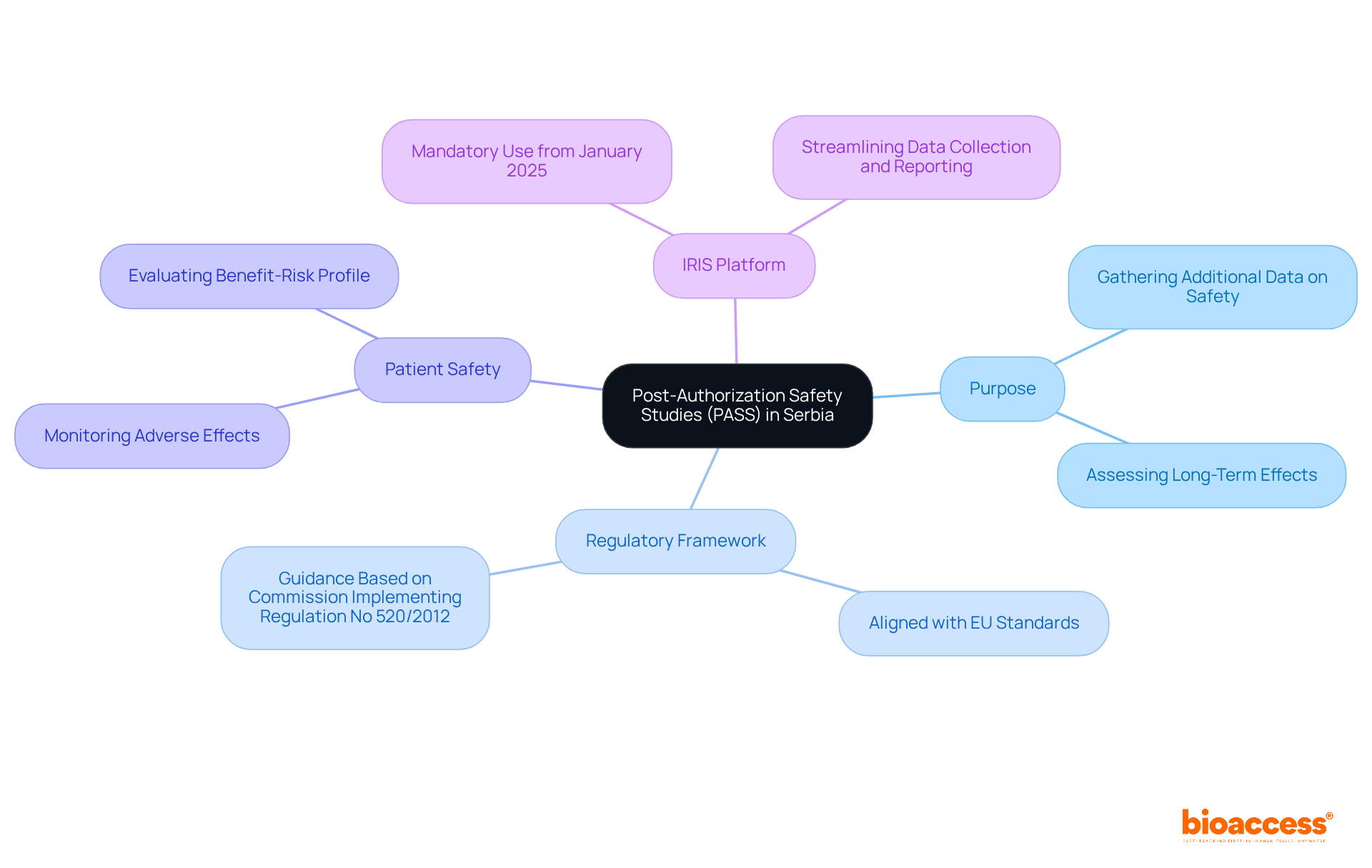
Implementing the post-authorization safety study requirements in Serbia is crucial for ensuring compliance with the regulatory standards set by the Agency for Medicines and Medical Devices of the region (ALIMS). Understanding the post-authorization safety study requirements in Serbia is essential for sponsors who aim to navigate the clinical research landscape effectively.
Before initiating a PASS, researchers must secure approval from a local ethics committee to comply with the post-authorization safety study requirements in Serbia. This committee meticulously examines the research framework to uphold ethical standards, reflecting Serbia's commitment to participant well-being and informed consent. The establishment of a Central Ethics Committee (CEC) further strengthens oversight and education regarding ethical dilemmas in clinical trials.
Thorough Research Framework: A comprehensive research framework is vital, detailing the project's objectives, methodology, and statistical analysis plan. It must also outline reporting methods for adverse events, ensuring that all potential risks are adequately managed. Bioaccess offers thorough reviews and feedback on study documents, ensuring compliance with country requirements and that protocols meet necessary standards.
Periodic Risk Reports: Sponsors are required to submit periodic risk reports to ALIMS, detailing any adverse events and the overall risk profile of the drug. Typically, these reports are submitted every six months for the first two years post-authorization, reinforcing the ongoing commitment to monitoring drug safety. Bioaccess assists in project management and monitoring, ensuring timely and accurate reporting, as well as support with import permits and nationalization of investigational devices.
Recent statistics reveal that most clinical trial applications in Serbia receive approval well within the 60-day review period, with some approvals finalized in as little as three weeks. Rory Gallagher notes that guidelines are often authorized within 30 days, and occasionally in just three weeks. This efficiency underscores the importance of local representatives in expediting the approval process and ensuring compliance with ALIMS guidelines for conducting risk assessments. Furthermore, anticipated regulatory updates in 2025 are expected to enhance the efficiency and effectiveness of ethical requirements, further improving the regulatory landscape.
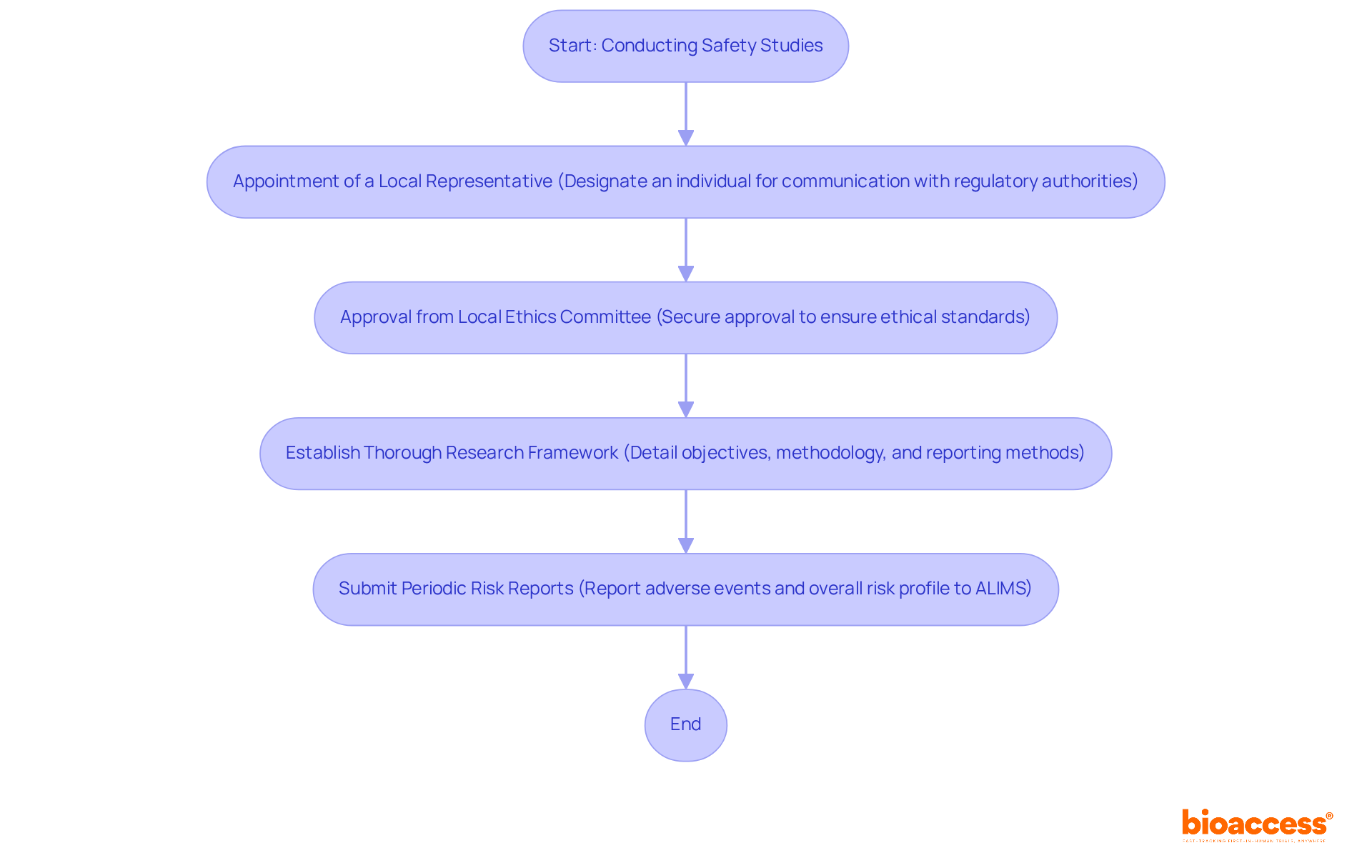
Implementing effective safety study protocols is crucial in clinical research, ensuring the integrity and safety of trials. Here are several key steps to consider:
Training and Team Coordination: Comprehensive training for all team members on study guidelines is essential. This ensures everyone understands their specific roles and responsibilities. Regular meetings should be scheduled to discuss progress, tackle challenges, and emphasize the importance of security protocols. Tailored training programs for clinical research teams in Serbia can significantly enhance team cohesion and operational efficiency while also addressing the post-authorization safety study requirements in Serbia.
Data Management Systems: Strong data management systems are vital for the effective gathering and analysis of security data. Electronic data capture (EDC) systems stand out for their efficiency, enabling real-time data entry and monitoring. This capability is crucial for preserving data integrity and adhering to regulatory guidelines.
Adverse Event Reporting: Establishing clear procedures for reporting adverse events is critical. This includes defining timelines for reporting serious adverse events (SAEs) and suspected unexpected serious adverse reactions (SUSARs). All team members must be well-versed in these procedures to ensure timely and accurate reporting, which is essential for patient safety and regulatory compliance.
Quality Assurance: Implementing quality assurance measures is necessary to monitor adherence to the protocol and regulatory requirements. Regular audits and evaluations of documentation help guarantee accuracy and completeness, fostering a culture of accountability and excellence in clinical research.
Stakeholder Communication: Maintaining open lines of communication with all stakeholders-including regulatory authorities, ethics committees, and research participants-is essential. This transparency builds trust and facilitates smoother study operations, ultimately enhancing the overall safety and efficacy of clinical trials while adhering to post-authorization safety study requirements in Serbia.

Post-Authorization Safety Studies (PASS) in Serbia play a crucial role in ensuring drug safety and efficacy in real-world settings. These studies not only adhere to European Union standards but also strengthen the overall pharmacovigilance framework, prioritizing patient well-being and informed decision-making in healthcare.
This article outlines three essential requirements for conducting PASS in Serbia:
These elements are vital for compliance with the Agency for Medicines and Medical Devices of Serbia (ALIMS) and for the successful execution of safety studies.
As the regulatory landscape evolves, particularly with the introduction of the IRIS platform in 2025, it is imperative for stakeholders to stay informed and adaptable. By embracing best practices and fostering collaboration among all parties involved, the integrity and efficacy of clinical research can be significantly enhanced. This ensures that patient safety remains at the forefront of drug development and monitoring efforts in Serbia.
What are Post-Authorization Safety Studies (PASS)?
Post-Authorization Safety Studies (PASS) are investigations conducted to gather additional safety data and assess the long-term effects of medications after they have received marketing authorization.
Why are PASS important in Serbia?
PASS are critical for pharmacovigilance as they help monitor adverse effects and evaluate the benefit-risk profile of approved medications, ensuring patient safety and well-being.
How does the regulatory framework for PASS in Serbia compare to other regions?
The regulatory framework governing PASS in Serbia is aligned with European Union standards, ensuring that studies are designed to effectively monitor medication safety.
What changes are expected for marketing authorization holders in Serbia by January 2025?
By January 2025, marketing authorization holders will be required to use the IRIS platform for managing PASS, which will streamline data collection and reporting processes.
What is the significance of using the IRIS platform for PASS?
The IRIS platform will enhance safety monitoring and support regulatory decision-making processes by improving the efficiency of data management in post-authorization safety studies.