


This article delineates three pivotal strategies for monitoring clinical trials, aimed at ensuring optimal compliance:
These strategies not only support compliance but also create a structured framework that prioritizes patient safety and accountability among team members. Furthermore, the integration of advanced technologies significantly improves data collection and oversight efficiency, reinforcing the importance of these strategies in the clinical research landscape.
In the intricate realm of clinical trials, compliance transcends mere regulatory obligation; it stands as a fundamental pillar of ethical research. By delineating precise monitoring objectives and harnessing cutting-edge technologies, research teams can markedly bolster data integrity and safeguard participant welfare. Yet, as the industry undergoes transformation, a pressing question arises: how can clinical trial teams adeptly navigate these changes while ensuring stringent oversight and accountability? This article delves into three pivotal strategies for optimizing compliance in clinical trials, offering insights poised to revolutionize the monitoring process and enhance study outcomes.
To establish efficient health assessment objectives, it is essential to first identify the primary aims of the research. These objectives may include ensuring patient safety, gathering high-quality information, and complying with regulatory standards. Once these aims are clearly defined, compliance standards should be established in accordance with relevant regulations, such as Good Clinical Practice (GCP) guidelines.
For instance, a research study aimed at evaluating a new biopharmaceutical should set goals that encompass how to monitor in clinical trials for adverse events and ensuring that data collection techniques are robust and reliable. By explicitly outlining these objectives and standards, the clinical research team can develop a targeted oversight plan to monitor in clinical trials, which addresses potential risks and guarantees adherence throughout the study.
Regularly reviewing and updating these objectives and standards as the experiment progresses can further enhance compliance and data integrity, which are vital to monitor in clinical trials. The recent revisions to GCP guidelines, particularly the ICH E6 R3 released in January 2025, underscore the necessity for a risk-proportionate approach and quality-by-design principles, which additionally strengthen the development of effective oversight practices. This framework not only safeguards participant welfare but also enhances the credibility of the results, aligning with the ethical standards articulated in the Declaration of Helsinki and the Nuremberg Code.
Moreover, targeted oversight plans can reduce on-site costs by approximately one-third, illustrating the tangible benefits of effective evaluation strategies. As noted by the World Medical Association, it is the physician's duty to advance and protect individual health, thereby reinforcing the ethical framework surrounding research studies. Furthermore, with around 70% of patient participants residing two hours away from their research sites, accessibility becomes a critical factor in monitoring the study.
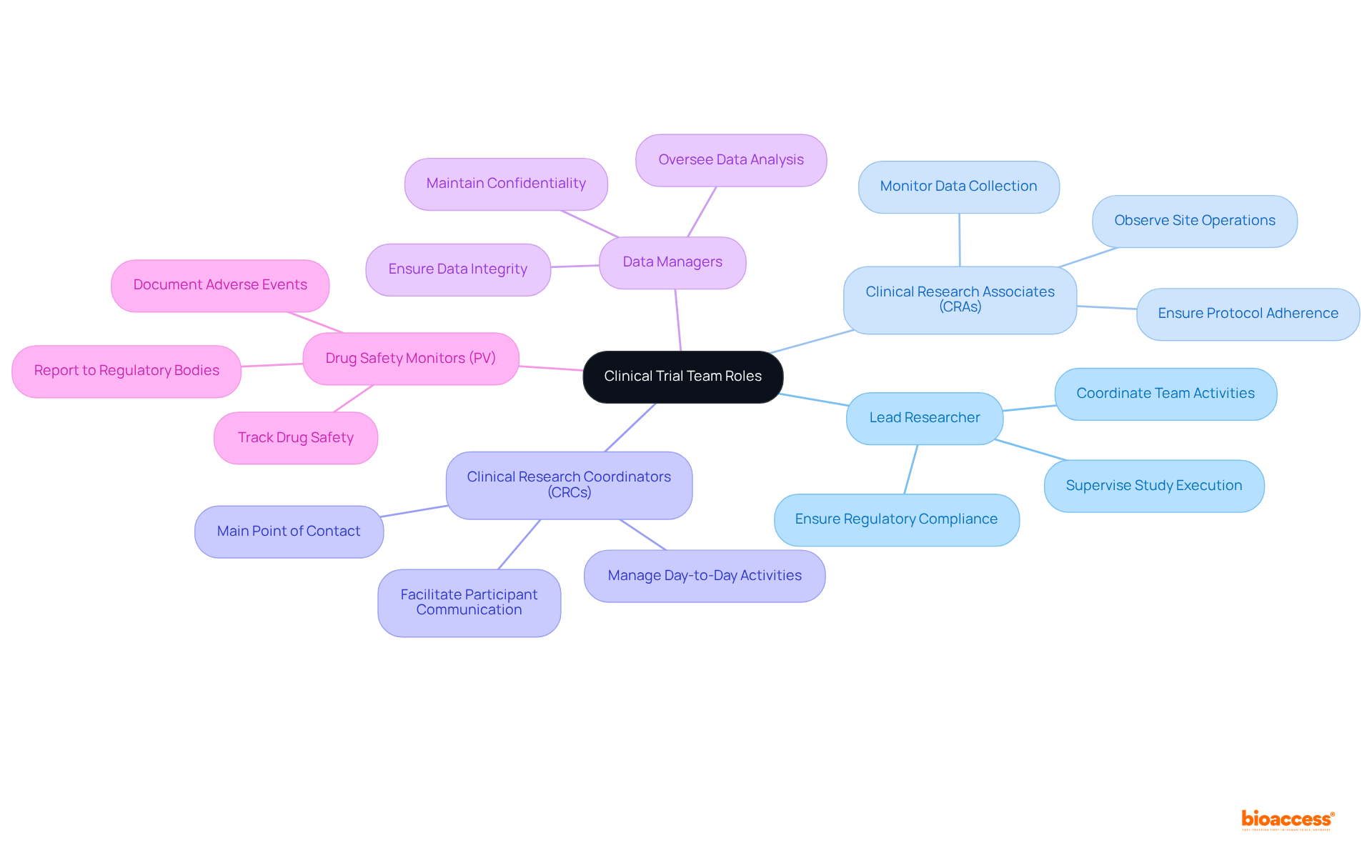
Clearly defining roles and responsibilities within the research team is crucial for fostering accountability and ensuring effective management. Every member, from the lead researcher to information managers and research associates (CRAs), must comprehend their individual responsibilities and how they contribute to the overall goals of the study.
For instance, the lead researcher supervises the study's execution and guarantees adherence to regulatory standards, while CRAs observe site operations and information gathering. Establishing a clear hierarchy and effective communication channels enables the team to collaboratively address compliance issues swiftly.
Frequent team gatherings and updates strengthen these roles, fostering a culture of responsibility that is vital for the success of research studies. As observed by Brian Achille, "Every research study necessitates a group of experts collaborating towards a shared objective: ensuring the safety of participants while producing precise and significant information."
Moreover, with only 5% of qualified patients engaging in research studies, the necessity for organized team dynamics becomes increasingly evident. Ambiguous roles can result in compliance challenges and impede patient recruitment.
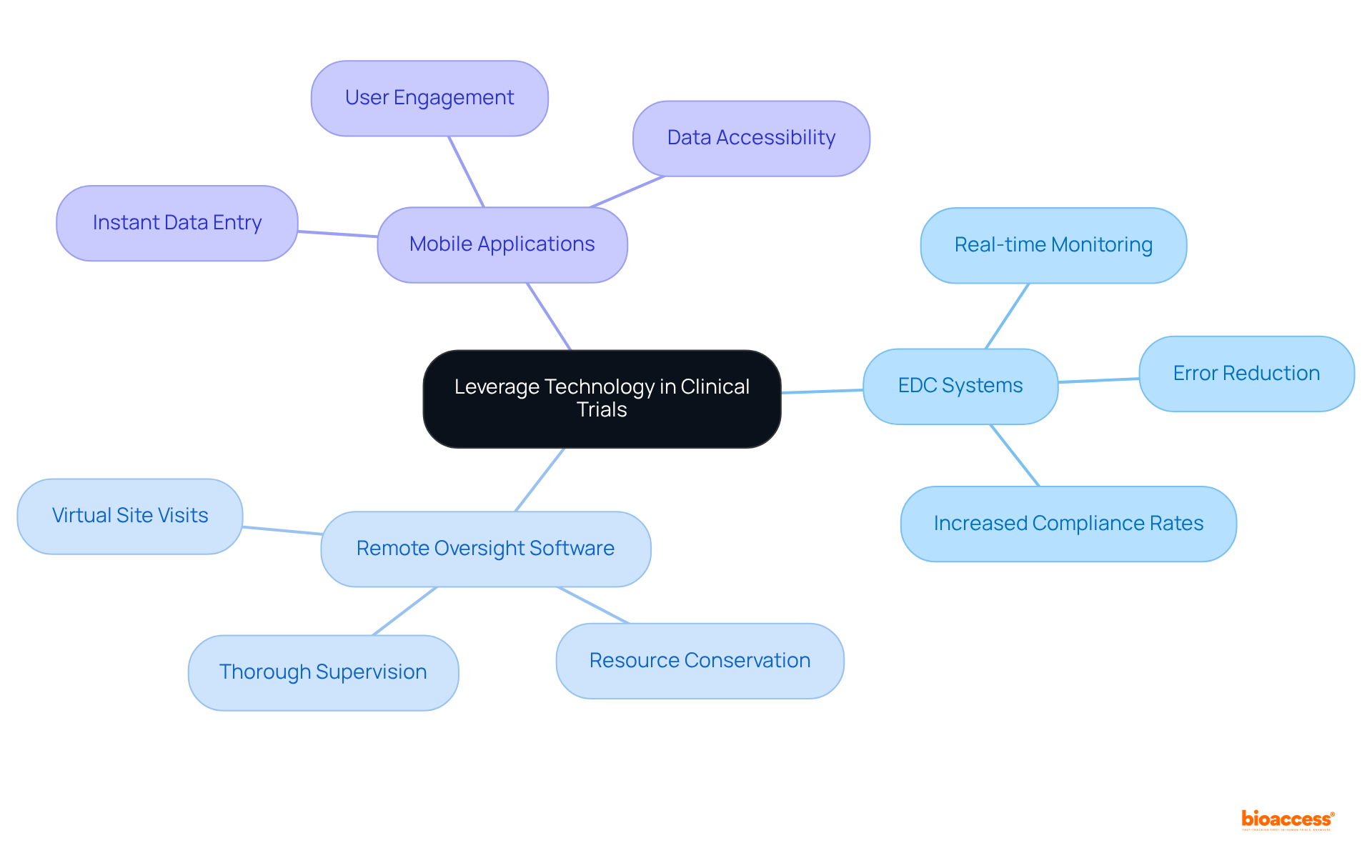
Incorporating technology to monitor in clinical trials is essential for significantly enhancing information integrity and adherence. Electronic information capture (EDC) systems, remote oversight software, and mobile applications facilitate real-time information collection and analysis, thereby reducing errors and improving overall efficiency. For instance, EDC systems enable instant information entry and validation, which streamlines the oversight process and ensures that discrepancies are promptly addressed. Furthermore, remote observation tools empower research associates (CRAs) to conduct virtual site visits, conserving time and resources while ensuring thorough supervision of study activities.
By leveraging these advanced technologies, research teams can bolster their ability to monitor in clinical trials, ensuring compliance with regulatory standards and elevating the quality of collected data. The adoption of EDC systems has been shown to enhance compliance rates; nearly 80% of research studies now utilize these systems to monitor in clinical trials, with numerous sites reporting increased confidence in data integrity thanks to automated workflows and real-time monitoring. As the research landscape evolves, the integration of these technologies becomes increasingly vital for improving study outcomes. As one industry specialist aptly noted, 'The days when research organizations could stay competitive without technology are long past.' Moreover, the clinical trial technology and services market is projected to reach USD 60.81 billion by 2030, highlighting the escalating significance of these innovations.
Establishing effective monitoring practices in clinical trials is essential for ensuring participant safety, data integrity, and regulatory compliance. By concentrating on clear objectives, defined roles, and the integration of advanced technology, research teams can significantly enhance their oversight capabilities. A well-structured approach not only protects participants but also strengthens the credibility of the research outcomes.
Las estrategias clave incluyen:
These elements work in concert to create a robust framework that enhances compliance and mitigates risks throughout the trial process.
In light of these insights, it is clear that prioritizing effective monitoring in clinical trials is vital for achieving high-quality results. As the landscape of clinical research continues to evolve, embracing these best practices will be crucial for ensuring the success of future studies. Stakeholders in the field are urged to adopt these strategies not only to improve compliance rates but also to contribute to the overall advancement of medical research.
What are the primary aims of establishing clinical monitoring objectives?
The primary aims include ensuring patient safety, gathering high-quality information, and complying with regulatory standards.
What guidelines should compliance standards align with?
Compliance standards should align with relevant regulations, such as Good Clinical Practice (GCP) guidelines.
Why is it important to outline objectives and standards in clinical research?
Clearly outlining objectives and standards allows the clinical research team to develop a targeted oversight plan, addressing potential risks and ensuring adherence throughout the study.
How can regular reviews of objectives and standards benefit clinical trials?
Regularly reviewing and updating objectives and standards can enhance compliance and data integrity, which are vital for monitoring in clinical trials.
What are the recent revisions to GCP guidelines mentioned in the article?
The recent revisions include the ICH E6 R3 released in January 2025, which emphasizes a risk-proportionate approach and quality-by-design principles.
How do effective oversight practices impact participant welfare and study credibility?
Effective oversight practices safeguard participant welfare and enhance the credibility of the results, aligning with ethical standards like the Declaration of Helsinki and the Nuremberg Code.
What financial benefits can targeted oversight plans provide?
Targeted oversight plans can reduce on-site costs by approximately one-third.
Why is accessibility a critical factor in monitoring clinical studies?
Accessibility is critical because around 70% of patient participants reside two hours away from their research sites, making it essential to consider their ability to participate in the study.