


Best practices for successful in vivo tests in clinical research encompass the utilization of appropriate methodologies, adherence to regulatory compliance and ethical standards, and the optimization of patient recruitment and retention strategies. This article elucidates these practices by detailing various methodologies, underscoring the significance of ethical guidelines and participant welfare, and outlining effective strategies for engaging participants. Each element is crucial for ensuring the reliability and success of clinical trials.
In the realm of clinical research, in vivo testing serves as a cornerstone for evaluating the safety and efficacy of new medical treatments. These experiments, conducted within living organisms, provide invaluable insights that in vitro methods cannot match, illuminating the complex interactions of treatments in real-world biological systems. As the demand for innovative therapies continues to rise, so does the imperative for best practices that ensure these tests are not only effective but also ethically sound.
What strategies can researchers implement to navigate the delicate balance between scientific advancement and ethical responsibility in in vivo studies?
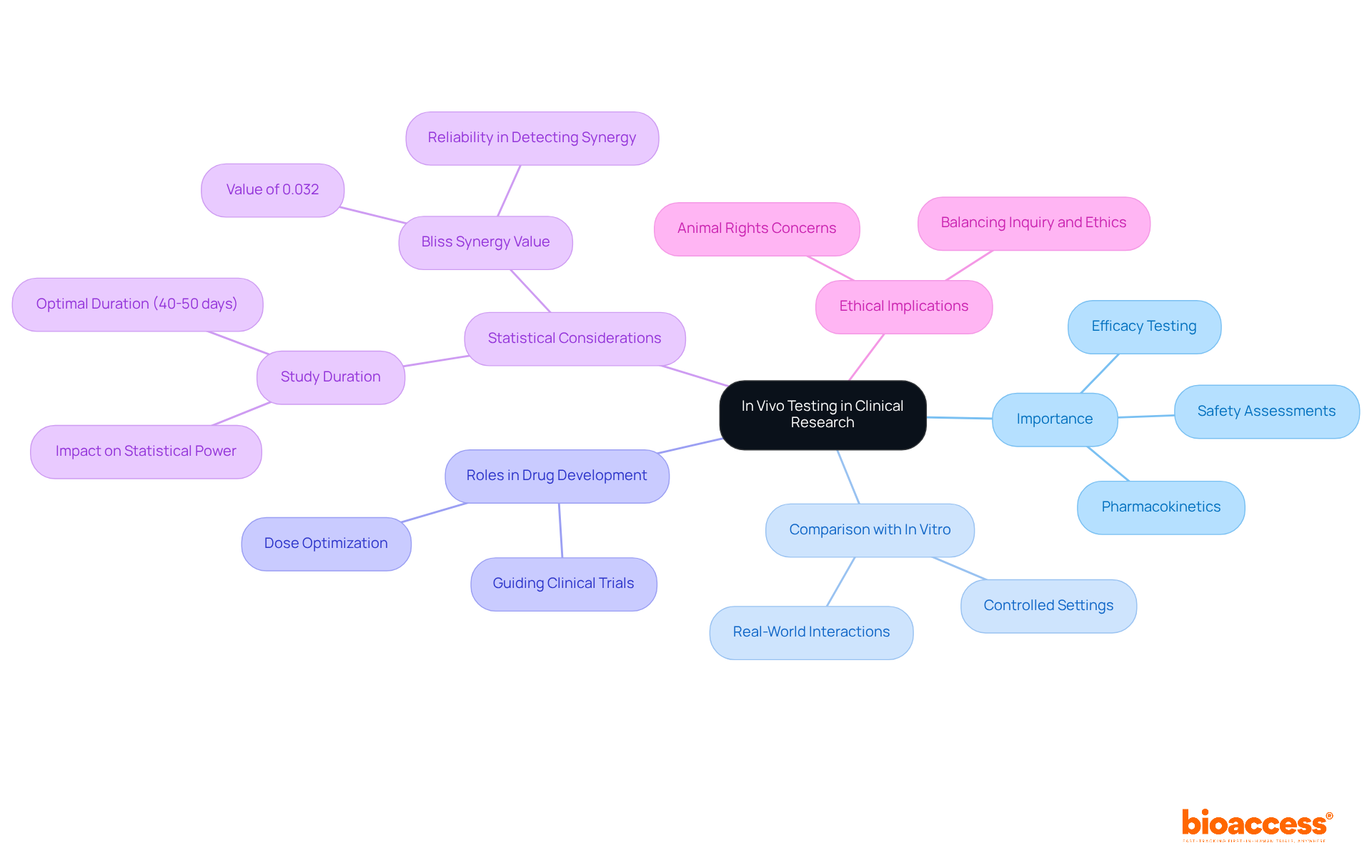
In a living organism, invivo tests refer to experiments conducted within animals or humans to evaluate the biological impacts of a treatment or intervention. This method is pivotal in clinical research, as it provides crucial insights into the efficacy, safety, and pharmacokinetics of new medical products.
Unlike in vitro experiments, which occur in controlled settings outside of living organisms, in situ evaluations enable researchers to observe the intricate interactions within a living system, rendering it essential for understanding how a treatment will function in real-world situations.
Invivo tests conducted in living organisms are integral to drug development, playing key roles in efficacy testing, safety assessments, and dose optimization, ensuring that new therapies are both effective and safe for human use. For instance, in the development of a new cancer therapy, invivo tests conducted in a living organism can reveal how the drug interacts with tumor cells, providing essential information that guides further clinical trials.
Statistics indicate that adequate statistical power to identify treatment effects is achieved with study durations of 40 to 50 days, particularly under the Bliss synergy model, which underscores the importance of well-structured in situ studies. Furthermore, the reliability of in situ evaluations is reinforced by recent discoveries demonstrating that invivo tests can effectively identify drug synergy, with a Bliss synergy value of 0.032, even under challenging circumstances.
This capability is crucial for advancing drug development, as it enables researchers to pinpoint promising treatment combinations that may enhance therapeutic outcomes in invivo tests. As we approach 2025, the emphasis on in situ evaluations will continue to grow, highlighting their essential role in the clinical study environment.
However, it is also vital to consider the ethical implications of in-life assessments, particularly regarding the use of sentient creatures, as emphasized by advocates for animal rights. Balancing the need for effective inquiry with ethical considerations remains a significant challenge in the field.
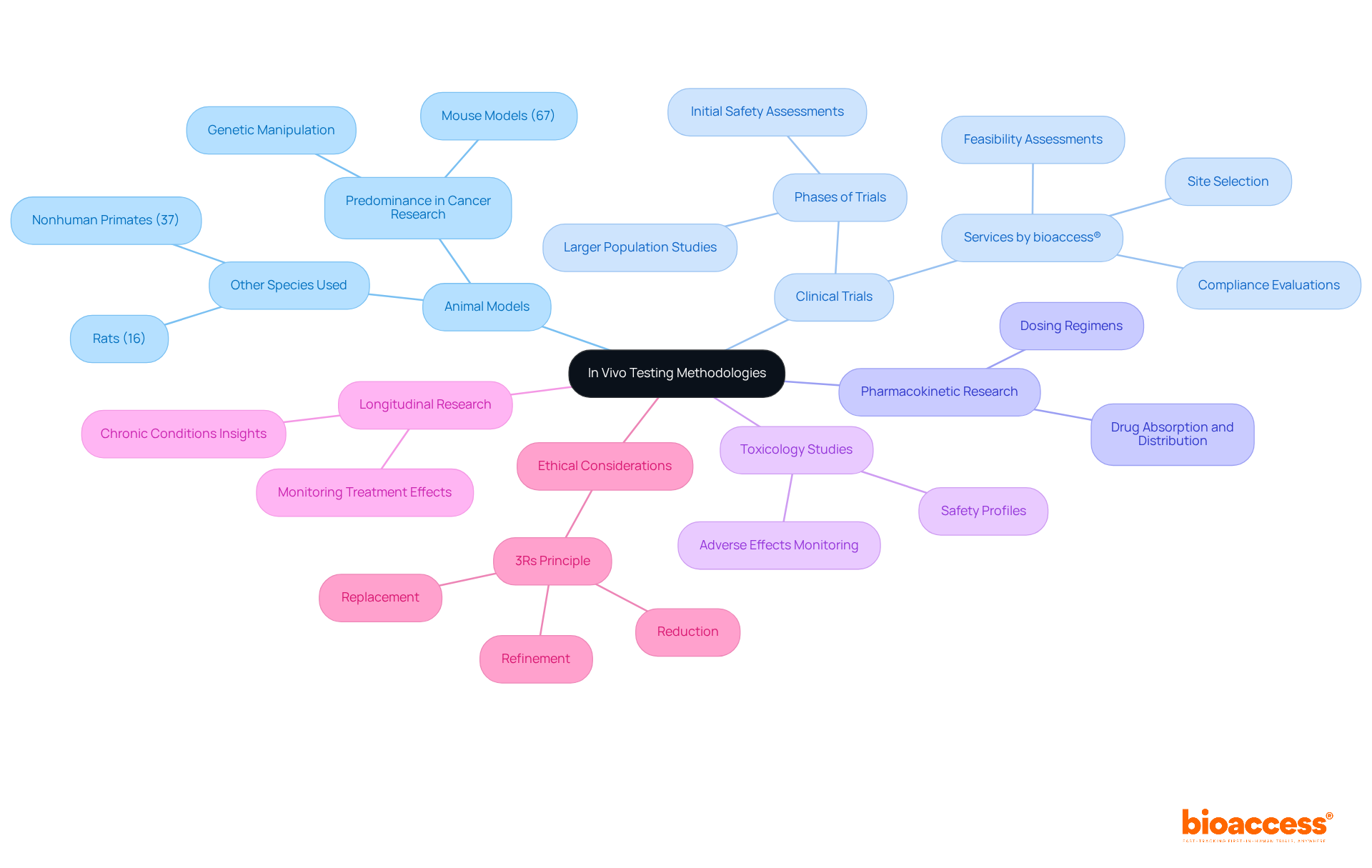
In vivo tests methodologies are varied and customized to the specific goals of each research project and the attributes of the product being assessed. Key methodologies include:
Animal Models: Various species are employed to replicate human disease conditions. For instance, mouse models are predominant in cancer research, used in approximately 67% of investigations to assess the efficacy of new therapies. Their genetic similarity to humans and the ability to manipulate their genomes make them invaluable for preclinical investigations.
Clinical Trials: These investigations are carried out directly with human participants to assess safety and efficacy. Clinical trials typically progress through phases, starting with small groups to establish safety before expanding to larger populations. bioaccess® focuses on overseeing these trials, providing services like feasibility assessments, site selection, compliance evaluations, and project management to guarantee a smooth process from beginning to end.
Pharmacokinetic Research: These investigations examine how a drug is absorbed, distributed, metabolized, and excreted in a living organism. Understanding these pharmacokinetic properties is essential for determining optimal dosing regimens and ensuring therapeutic effectiveness during invivo tests. bioaccess® offers expertise in carrying out these evaluations, especially in the context of early-feasibility and pivotal assessments, to assist U.S. medical device firms in Colombia.
Toxicology Studies: Safety profiles of new drugs are assessed by observing adverse effects in animal models prior to human trials. This step is crucial for identifying potential risks and ensuring participant safety in subsequent clinical phases, particularly during invivo tests. bioaccess® guarantees strict adherence and comprehensive reporting in these analyses, improving the dependability of results.
Longitudinal Research: These investigations monitor the effects of a treatment over prolonged durations, offering insights into long-term efficacy and safety. Such research is essential for comprehending chronic conditions and the lasting effects of therapies. With bioaccess®'s tailored approach, clients can navigate the complexities of these analyses effectively.
Each approach offers distinct strengths and weaknesses, and the choice should correspond with the particular objectives of the study. While animal models offer critical insights into biological processes, they may not always accurately predict human responses, underscoring the need for careful interpretation of results. Recent trends indicate a growing reliance on genetically engineered models, which have shown improved fidelity in mimicking human diseases, thus enhancing the relevance of preclinical findings. Furthermore, commitment to the 3Rs (Replacement, Reduction, Refinement) principle is vital for ethical animal experimentation, guaranteeing that investigations are carried out responsibly.
Ensuring regulatory compliance and ethical standards in live testing is vital for participant protection and the integrity of research outcomes. Key considerations include:
By following these standards, investigators can enhance the reliability of their studies and cultivate public confidence in clinical investigations, ultimately aiding the advancement of medical knowledge.

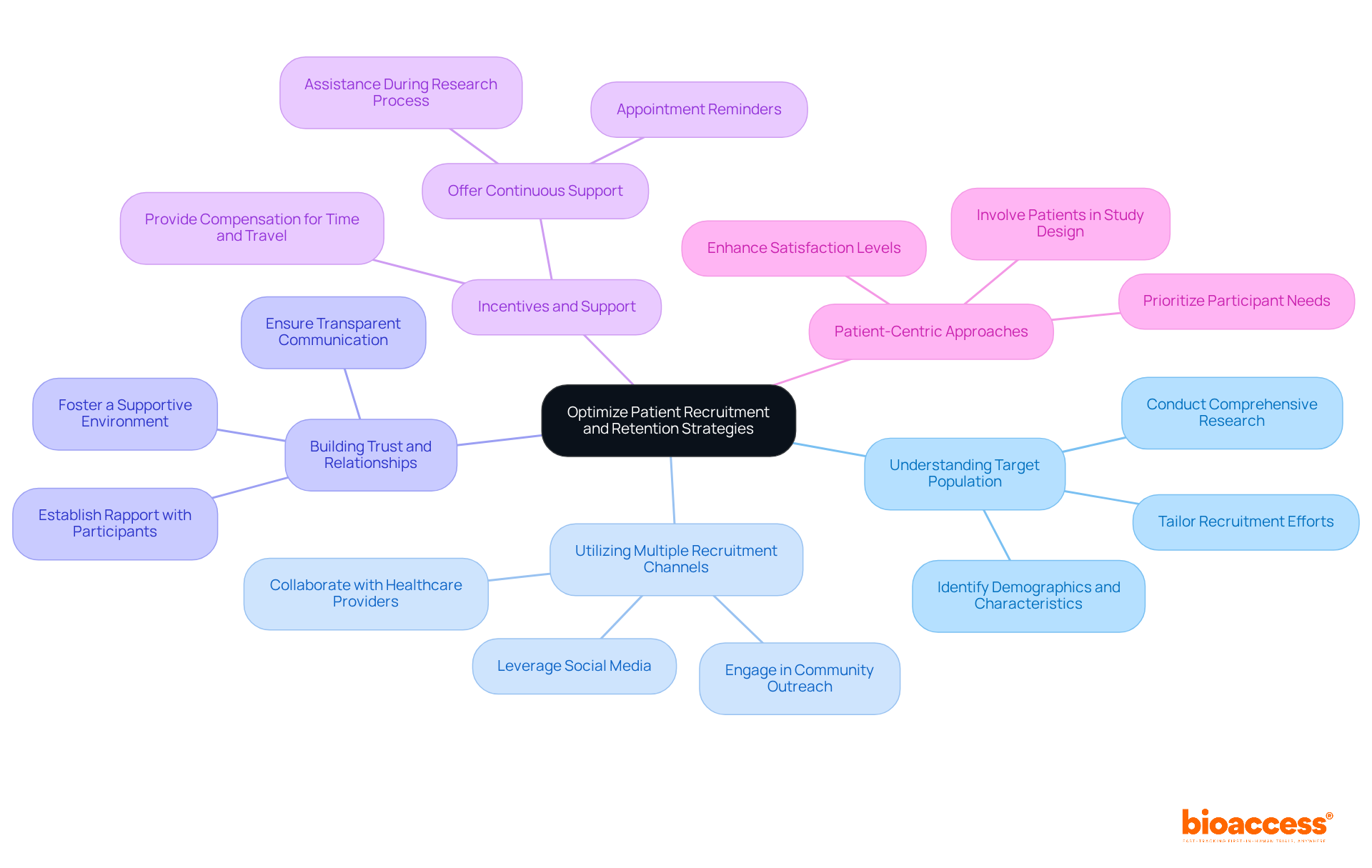
Optimizing patient recruitment and retention is crucial for the success of invivo tests. Effective strategies to enhance these processes are essential for researchers aiming to improve clinical trial outcomes.
Understanding the target population is fundamental. Comprehensive research should be conducted to identify the demographics and characteristics of potential participants. Tailoring recruitment efforts based on these insights significantly improves engagement and participation rates.
Utilizing multiple recruitment channels broadens the reach. Employing a variety of platforms, including social media, community outreach, and collaborations with healthcare providers, attracts a diverse participant pool, enhancing the overall study quality.
Building trust and relationships is vital. Establishing rapport with potential participants enhances their willingness to enroll. This can be achieved through transparent communication, addressing concerns, and fostering a supportive environment that encourages participation.
Incentives and support play a critical role in motivation. Providing compensation for time and travel can encourage participation. Furthermore, continuous support during the research process, including appointment reminders and assistance, significantly enhances retention rates.
Finally, patient-centric approaches ensure that participant needs are prioritized. Involving patients in the study design process leads to higher satisfaction levels and reduces dropout rates.
By implementing these strategies, researchers can enhance participant engagement, ultimately leading to more successful and reliable invivo tests.
In vivo testing serves as a cornerstone of clinical research, providing invaluable insights into the efficacy and safety of new medical treatments within living organisms. This method is essential not only for understanding biological interactions but also for ensuring that therapies are effective and safe for human use. As the clinical research landscape evolves, the significance of well-structured in vivo tests will continue to grow, reinforcing their pivotal role in advancing medical science.
The article delineates several best practices for successful in vivo tests, including:
Collectively, these practices contribute to a more rigorous and ethical research environment, ensuring that new therapies can be developed responsibly.
Ultimately, the advancement of clinical research hinges on a steadfast commitment to these best practices. By prioritizing ethical considerations, employing diverse methodologies, and focusing on participant engagement, researchers can significantly enhance the quality and impact of in vivo tests. Embracing these strategies not only fosters trust in the research process but also paves the way for innovative treatments that can improve patient outcomes in the future.
What is in vivo testing in clinical research?
In vivo testing refers to experiments conducted within living organisms, such as animals or humans, to evaluate the biological impacts of a treatment or intervention.
How does in vivo testing differ from in vitro testing?
In vivo testing occurs within living organisms, allowing researchers to observe interactions within a living system, while in vitro testing takes place in controlled environments outside of living organisms.
Why are in vivo tests important in drug development?
In vivo tests are crucial for assessing the efficacy, safety, and pharmacokinetics of new medical products, ensuring that therapies are effective and safe for human use.
Can you provide an example of in vivo testing in action?
In the development of a new cancer therapy, in vivo tests can reveal how the drug interacts with tumor cells, providing essential information that guides further clinical trials.
What is the significance of study duration in in vivo testing?
Adequate statistical power to identify treatment effects is typically achieved with study durations of 40 to 50 days, particularly under the Bliss synergy model.
How do in vivo tests contribute to identifying drug synergy?
In vivo tests can effectively identify drug synergy, with a Bliss synergy value indicating potential promising treatment combinations that may enhance therapeutic outcomes.
What ethical considerations are associated with in vivo testing?
Ethical implications arise from the use of sentient creatures in research, highlighting the need to balance effective inquiry with ethical considerations, as emphasized by animal rights advocates.