


The article highlights the significance and regulatory framework of FDA medical device Class 1 products, which are classified as low-risk and account for nearly 50% of healthcare instruments in the U.S. This classification underscores their essential role in patient care, coupled with a streamlined approval process. These devices are subject to fewer regulations, facilitating quicker market entry while still adhering to stringent safety standards. Understanding this landscape is crucial for stakeholders in clinical research, as it not only reflects the current state of Medtech but also the opportunities and challenges that lie ahead.
Understanding the landscape of FDA medical device regulations is paramount for manufacturers navigating the complexities of healthcare compliance.
How can manufacturers ensure they remain compliant and competitive in this dynamic landscape?
Health products classified as FDA medical device class 1 are considered low-risk items, subject to the least regulatory supervision. These products are not designed to support or sustain life, nor are they critical in preventing impairment. Common examples of items categorized as FDA medical device class 1 include:
The significance of FDA medical device Class 1 instruments is highlighted by their widespread presence in medical environments, where they play a crucial role in patient care while adhering to strict safety and efficacy standards. Notably, nearly 50% of healthcare instruments in the U.S. market are categorized as FDA medical device class 1, emphasizing their vital role in daily healthcare practice. This categorization as FDA medical device class 1 facilitates quicker market entry, fostering innovation within the equipment industry and ensuring that providers have prompt access to essential tools. As we approach 2025, the influence of FDA medical device Class 1 instruments remains substantial, aiding a broad spectrum of medical activities and enhancing patient outcomes. Expert insights reveal that despite being categorized as FDA medical device class 1, these instruments are indispensable in delivering quality care and improving the overall efficiency of healthcare systems. Furthermore, products classified as FDA medical device Class 1 are governed by general regulations under the FD&C Act, which ensures their safety and effectiveness in the marketplace.
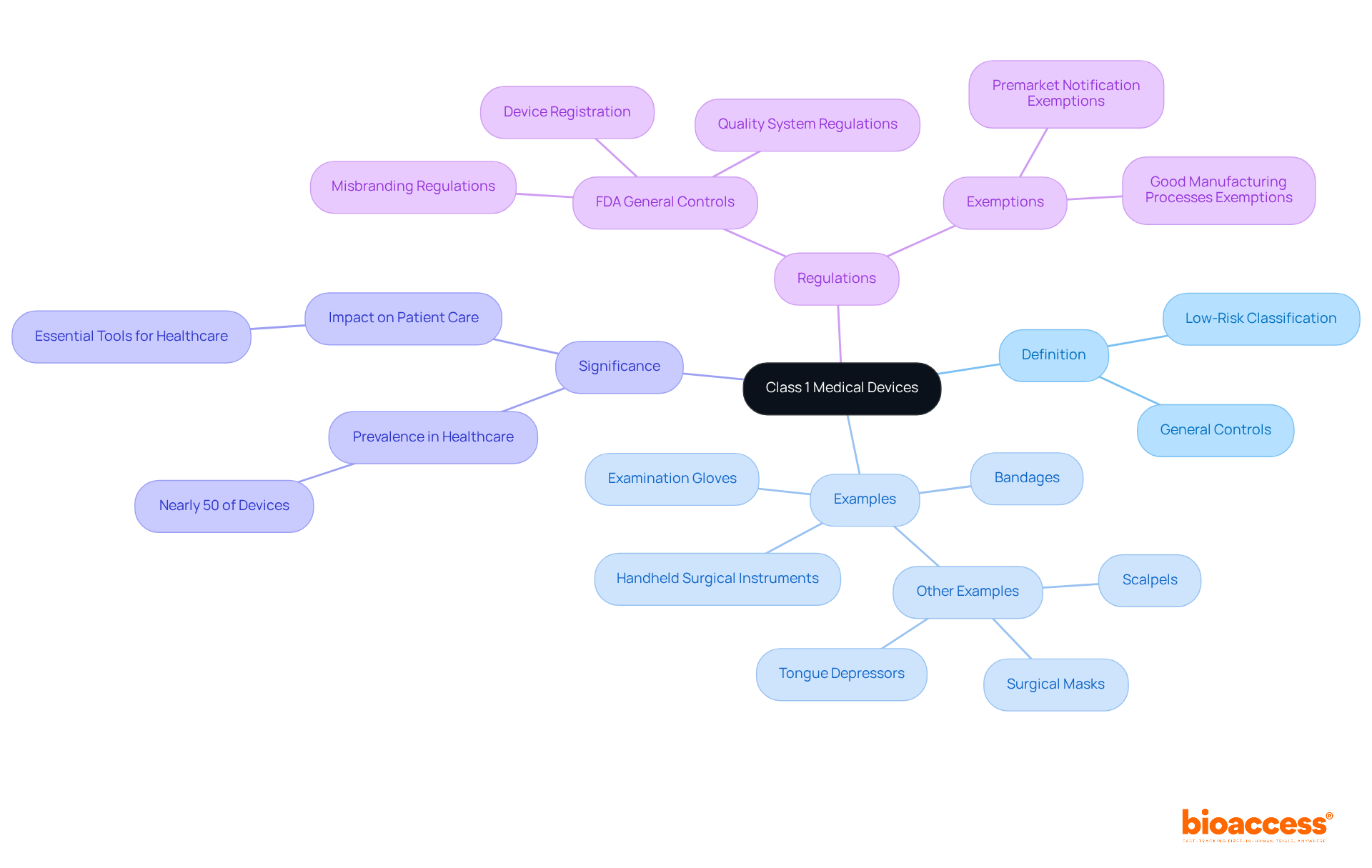
The FDA medical device class 1 products typically benefit from exemptions to the Premarket Notification 510(k) requirements, allowing them to bypass the extensive premarket review mandated for higher-risk items. However, manufacturers must comply with general controls, which include:
Notably, exemptions can differ based on the equipment's intended use and specific characteristics; for example, equipment designed to prevent significant health impairment may not qualify for these exemptions. According to FDA statistics, approximately 35% of regulated items fall into the FDA medical device class 1 category, underscoring the importance of understanding these regulatory nuances.
Manufacturers can effectively navigate these complexities by utilizing resources such as the FDA's Product Classification database to verify exemption statuses and ensure compliance with relevant regulations. Staying informed about the latest FDA recommendations is crucial, especially with the upcoming exclusion of certain unclassified health products from premarket notification obligations starting July 1, 2025. This evolving landscape demands vigilance to avoid potential pitfalls in the approval process.
At Bioaccess, we provide a comprehensive approach to advancing healthcare equipment trials, which includes:
Our expertise in project management and oversight addresses the challenges faced by health technology startups, such as regulatory hurdles, competition, recruitment issues, and financial constraints. With professionals like Ana Criado, our Director of Regulatory Affairs, who possesses extensive experience in biomedical engineering and regulatory consulting, we are well-equipped to guide manufacturers through the complexities of the approval process.
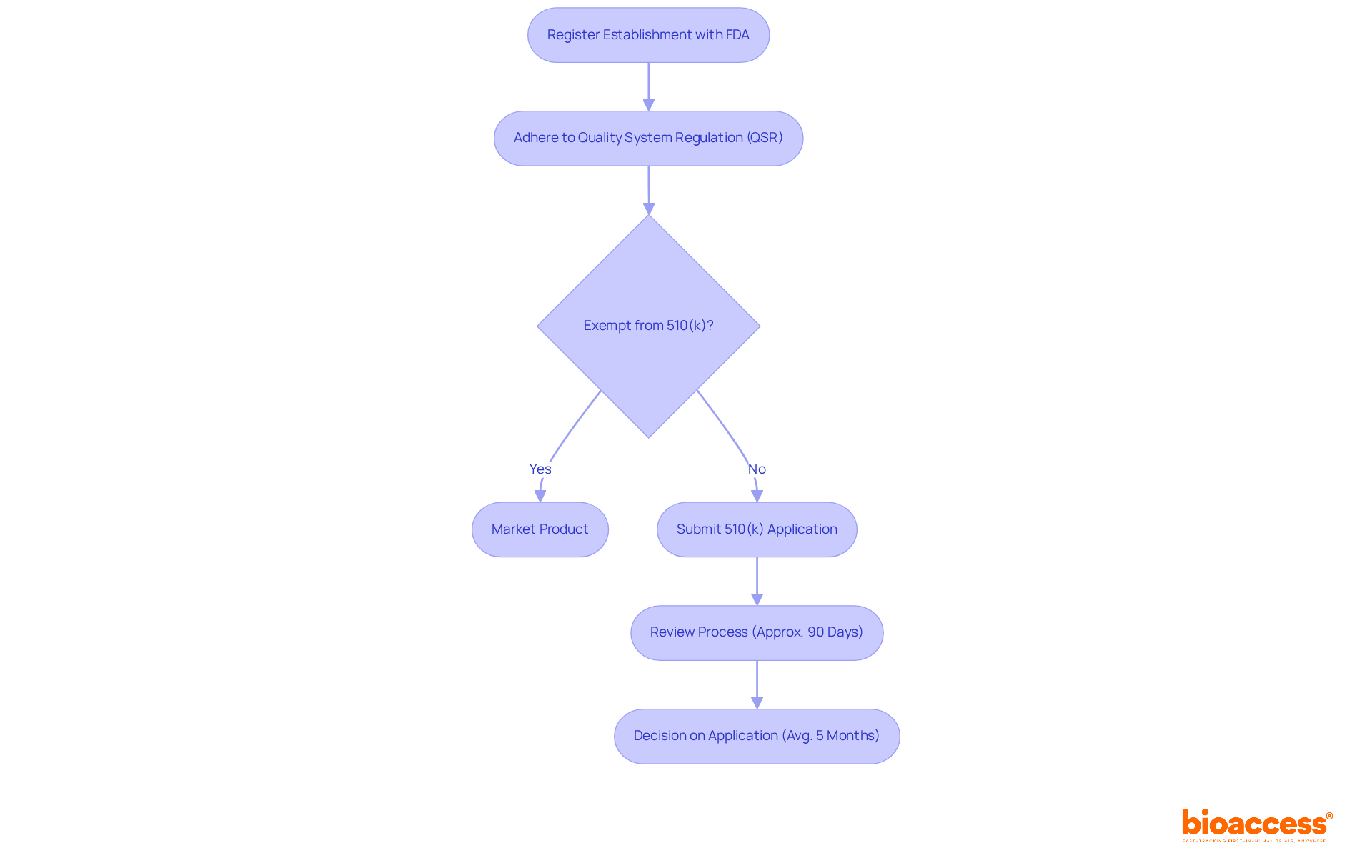
The approval procedure for FDA medical device class 1 products is notably simpler than that for higher categories, making it an attractive option for producers. Initially, manufacturers must register their establishment with the FDA and adhere to the Quality System Regulation (QSR). Most products classified as FDA medical device class 1 enjoy exemptions from the 510(k) requirements, allowing them to be marketed once they meet all general controls.
However, for those that are not exempt, a 510(k) submission is essential to demonstrate substantial equivalence to a legally marketed product. This submission process typically spans about 90 days, although timelines can vary significantly based on the complexity of the submission and the FDA's review workload. In fact, the average time for a decision on 510(k) applications approaches five months, with 85 percent receiving a Substantially Equivalent decision by September 2022.
Understanding these nuances is crucial for ensuring prompt market entry and effective commercialization of FDA medical device Class 1 products. Effective market entry strategies often involve proactive communication with the FDA, which can enhance submission quality and reduce review times. Successful instances include manufacturers who have adeptly navigated the self-registration process, commonly completing it within one week, thereby expediting their path to market.
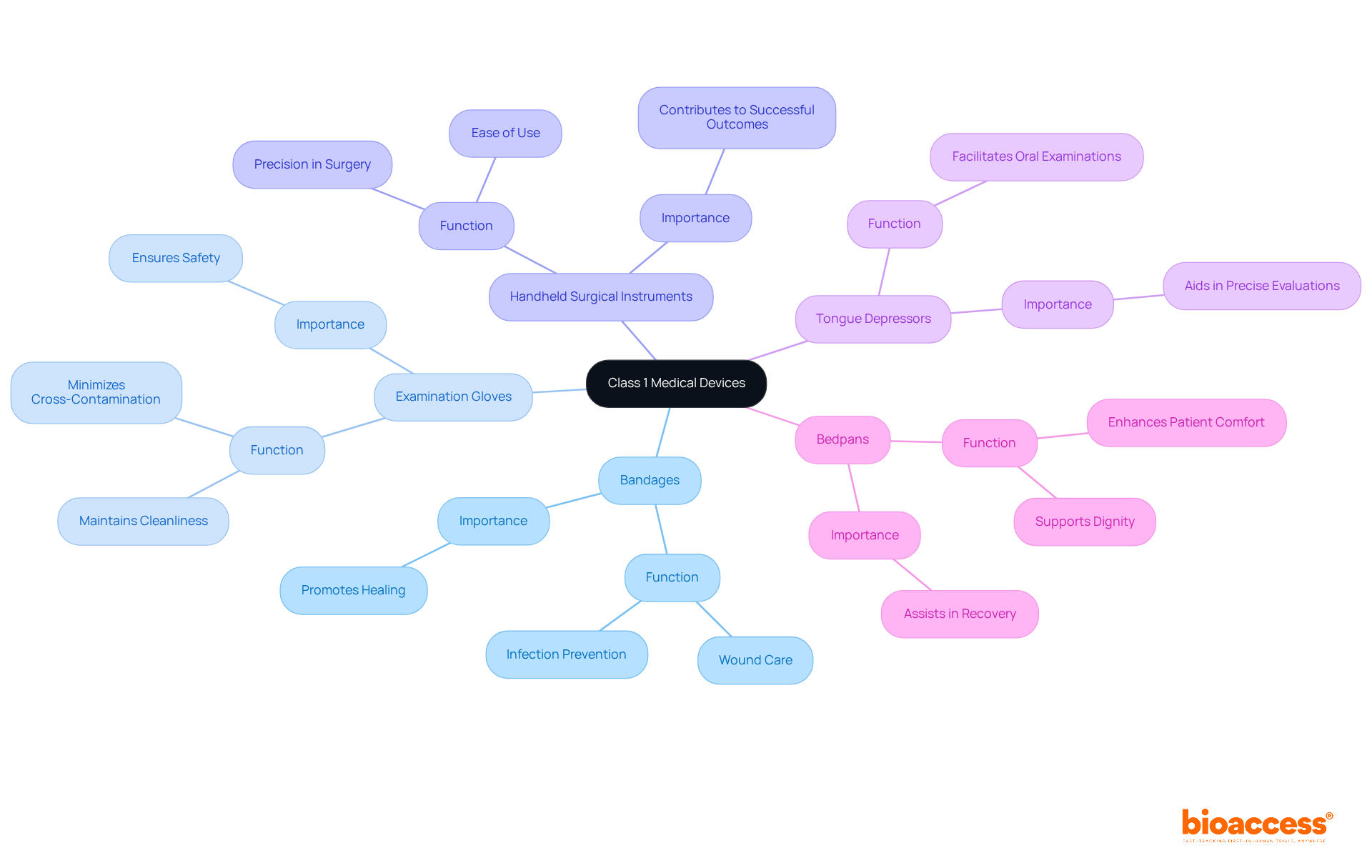
Class 1 healthcare devices, which are categorized as FDA medical device class 1, encompass a variety of essential tools that play a pivotal role in patient care. Notable examples include:
These examples demonstrate the essential function of FDA medical device Class 1 instruments in healthcare, highlighting their low-risk characteristics while emphasizing their significance in patient care and safety. Products classified as FDA medical device class 1 represent around 47% of all healthcare equipment, with 95% being free from regulations due to their low risk. Recent innovations in this category, including advancements in materials and design, continue to enhance their effectiveness and usability, further solidifying their place in modern medical practice. As noted by the MDCG, compliance with safety and performance requirements is essential for these devices, ensuring they meet the necessary standards for patient safety.
The landscape of FDA medical device Class 1 regulations reveals a framework that is both essential and accessible for manufacturers of low-risk healthcare products. By understanding the classification and regulatory environment, stakeholders can appreciate the critical role these devices play in patient care and the healthcare system at large. Class 1 medical devices, while often overlooked due to their low-risk designation, form the backbone of everyday medical practices, ensuring safety and efficiency in patient treatment.
Key insights into the regulatory requirements, exemptions, and approval processes for Class 1 devices underscore the importance of compliance and strategic planning for manufacturers. The ability to navigate the FDA's guidelines, from understanding the significance of general controls to leveraging exemptions, is vital for ensuring timely market entry and maintaining product quality. Furthermore, the examples of commonly used Class 1 devices highlight their indispensable role in healthcare, reinforcing the notion that even low-risk items contribute significantly to patient safety and care.
In conclusion, as the regulatory landscape evolves, particularly with the upcoming changes in 2025, it becomes increasingly important for manufacturers and healthcare providers to stay informed and proactive. Emphasizing the significance of FDA medical device Class 1 instruments not only enhances operational efficiency but also ultimately leads to improved patient outcomes. Engaging with resources and expertise in regulatory affairs can empower stakeholders to navigate these complexities effectively, ensuring that essential medical devices continue to support quality care in clinical settings.
What are Class 1 medical devices?
Class 1 medical devices are health products classified by the FDA as low-risk items, subject to the least regulatory supervision. They are not designed to support or sustain life, nor are they critical in preventing impairment.
Can you provide examples of Class 1 medical devices?
Common examples of Class 1 medical devices include bandages, examination gloves, and handheld surgical instruments.
Why are Class 1 medical devices important in healthcare?
Class 1 medical devices play a crucial role in patient care and are widely used in medical environments. They adhere to strict safety and efficacy standards, making them vital for daily healthcare practice.
What percentage of healthcare instruments in the U.S. market are Class 1 medical devices?
Nearly 50% of healthcare instruments in the U.S. market are categorized as FDA medical device Class 1.
How does the classification of Class 1 medical devices affect market entry?
The classification as FDA medical device Class 1 facilitates quicker market entry, promoting innovation within the equipment industry and ensuring that providers have prompt access to essential tools.
What regulations govern Class 1 medical devices?
Products classified as FDA medical device Class 1 are governed by general regulations under the FD&C Act, which ensures their safety and effectiveness in the marketplace.
What is the future outlook for Class 1 medical devices as we approach 2025?
As we approach 2025, the influence of FDA medical device Class 1 instruments remains substantial, aiding a broad spectrum of medical activities and enhancing patient outcomes.