


This article delves into crucial insights pertinent to scenarios where the sponsor investigator possesses the Investigational New Drug (IND) application. It underscores the necessity of prompt ethical approvals and adept management of clinical holds. By leveraging regulatory expertise and ensuring compliance, organizations can effectively navigate the complexities of clinical research, streamlining the IND application process. This approach not only enhances efficiency but also addresses the pressing challenges faced in the Medtech landscape. Collaboration and strategic action are essential for success in this intricate field.
Navigating the complex landscape of Investigational New Drug (IND) applications can be overwhelming, particularly when the sponsor investigator plays a pivotal role in the process. Securing rapid ethical approvals is not merely about speed; it’s a crucial element that can significantly influence the success of innovative therapies reaching the market. As clinical trials encounter heightened scrutiny and regulatory hurdles, grasping the intricacies of clinical holds becomes vital for sponsors. What strategies can organizations implement to not only circumvent these holds but also guarantee compliance and efficiency in their IND applications?
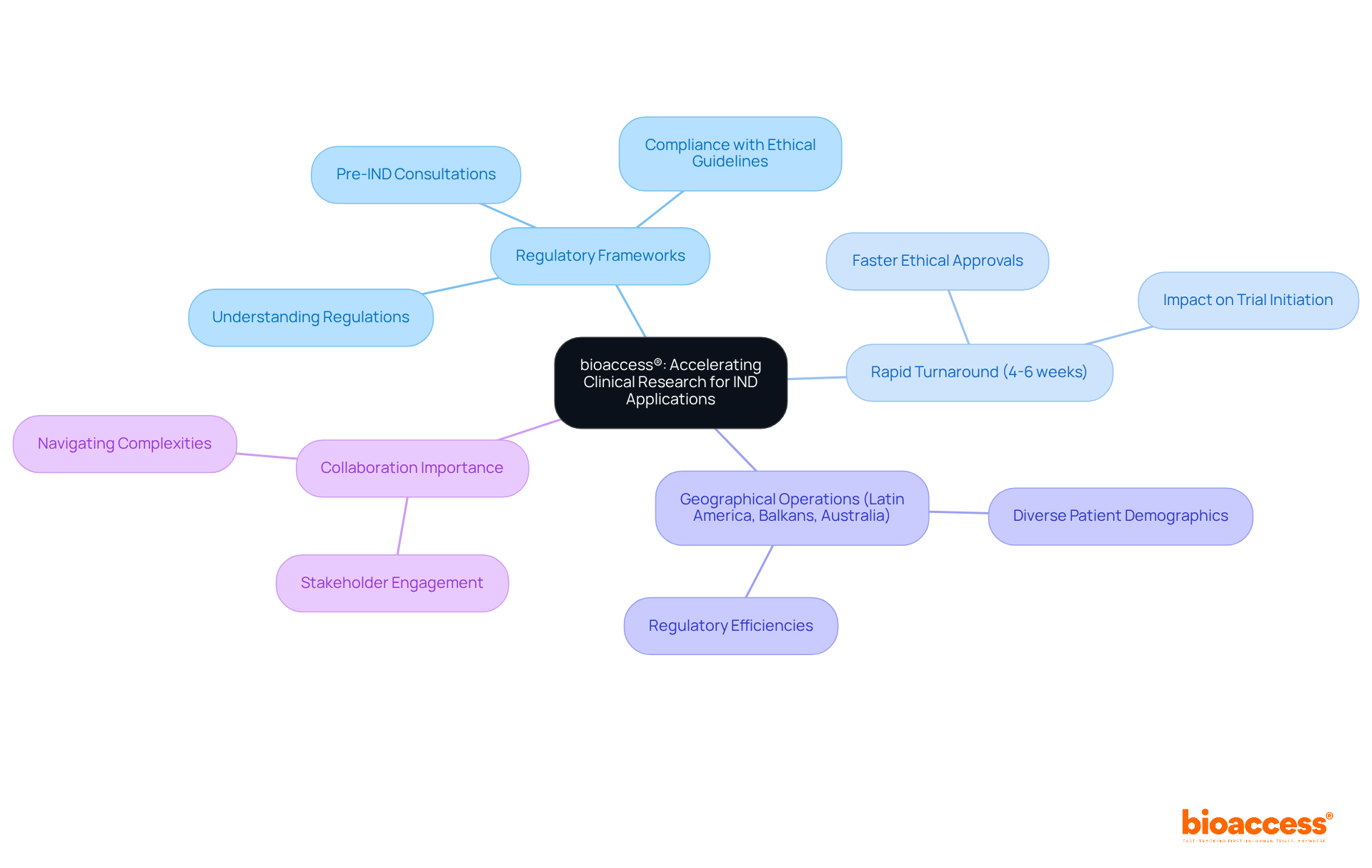
bioaccess® stands out in simplifying the IND application process, thanks to its deep understanding of regulatory frameworks and research environments. By specializing in early-phase studies, bioaccess® secures ethical approvals in an impressive 4-6 weeks—significantly faster than traditional timelines. This rapid turnaround is crucial for sponsors eager to initiate trials when the sponsor investigator holds the IND, ultimately accelerating the journey to market for innovative therapies.
With operations spanning Latin America, the Balkans, and Australia, bioaccess® leverages diverse patient demographics and regulatory efficiencies, establishing itself as a preferred partner for Medtech, Biopharma, and Radiopharma innovators. Recent advancements in IND applications underscore the growing importance of swift ethical approvals, particularly when the sponsor investigator holds the IND, as organizations increasingly recognize the need for agility in research to meet evolving market demands.
In this dynamic landscape, collaboration becomes essential. By partnering with bioaccess®, stakeholders can navigate the complexities of clinical research more effectively, ensuring that groundbreaking therapies reach those who need them most.
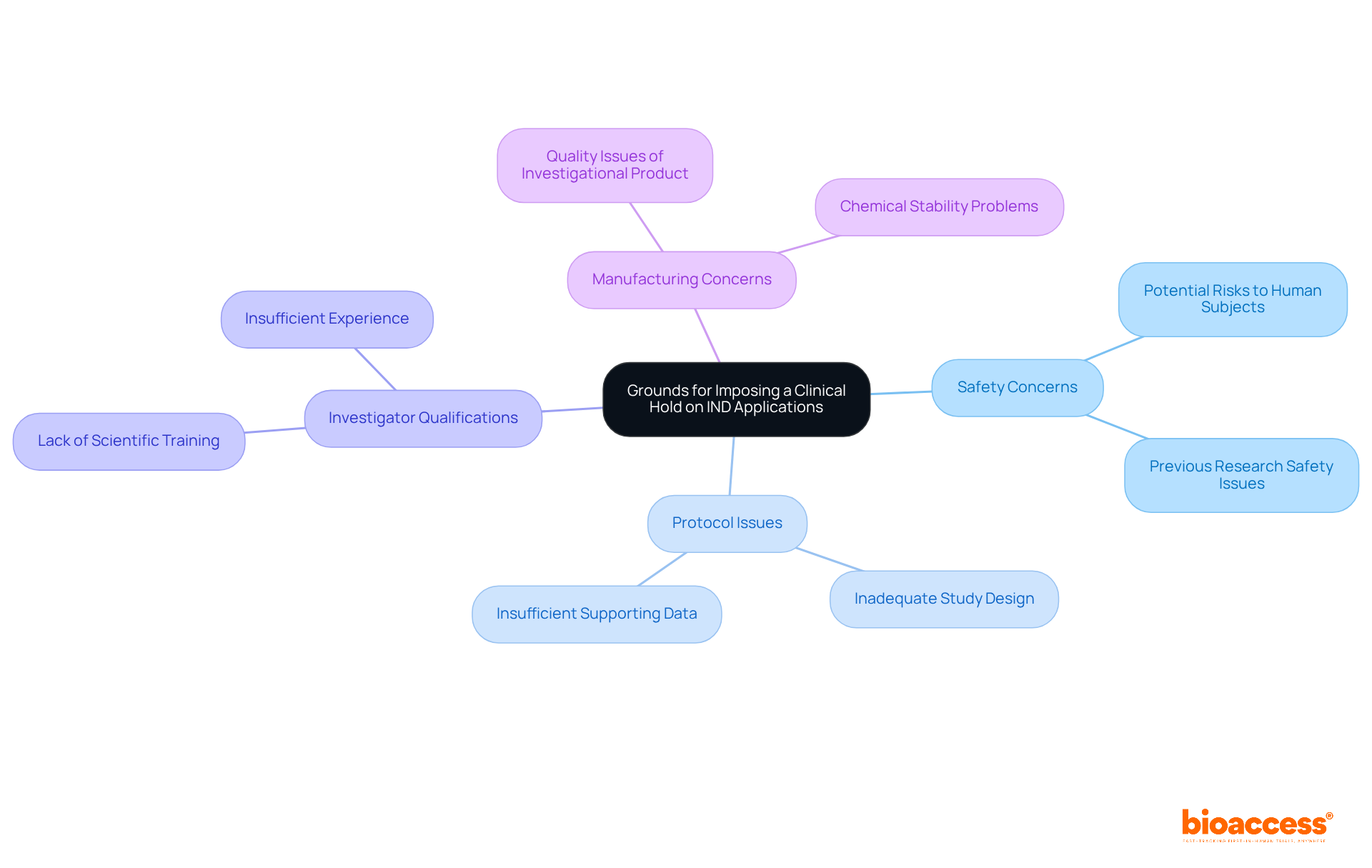
The FDA may impose a clinical hold on an IND application for several critical reasons:
Grasping these foundations is crucial for sponsors to guarantee that their IND applications are strong and adhere to compliance expectations. Involving compliance specialists, like Ana Criado and Katherine Ruiz, early in the IND application phase can assist in recognizing potential issues and simplifying the approval process. Their knowledge in compliance matters and trial management services—including feasibility studies, site selection, compliance evaluations, trial setup, import permits, project management, and reporting—is invaluable as the FDA seeks to address deficiencies with the IND applicant before issuing a trial suspension order unless there is urgent serious risk to patients.
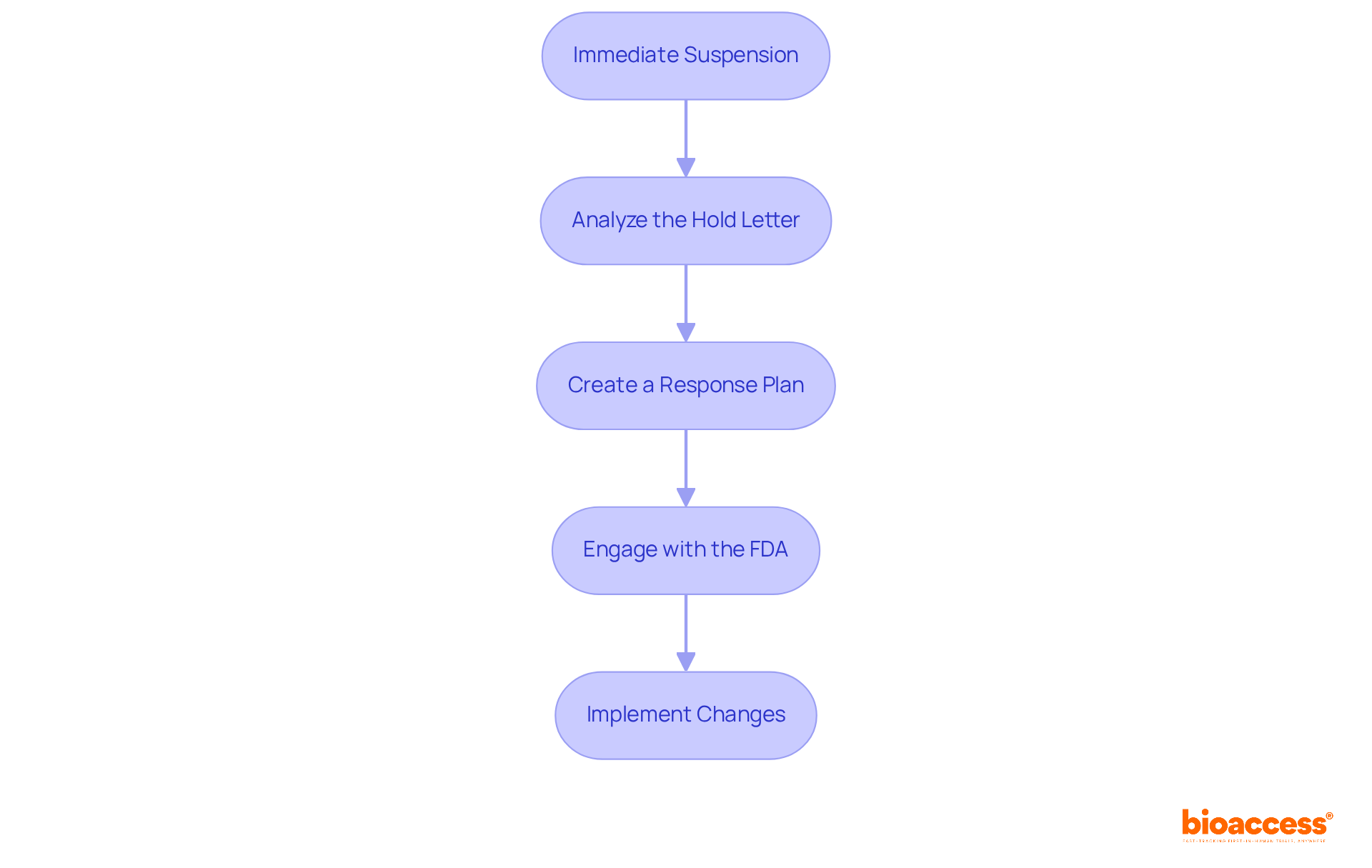
When a clinical hold is imposed, sponsors must adhere to the following procedures to effectively manage the situation:
By following these steps, sponsors can navigate the complexities of research pauses more efficiently, thereby minimizing disruptions to trial schedules. It's noteworthy that only about 10% of medications entering trials receive FDA approval, underscoring the importance of a robust response strategy to trial delays. As compliance advisors emphasize, 'A thoroughly prepared response strategy can significantly enhance the likelihood of resolving a trial suspension and advancing with the study.' Furthermore, proactive engagement with the FDA, such as through Pre-IND meetings, can lead to improved outcomes in managing regulatory suspensions. Leveraging the expertise of specialists like Katherine Ruiz, a recognized authority in compliance matters for medical devices and in vitro diagnostics in Colombia, can further strengthen the response strategy.
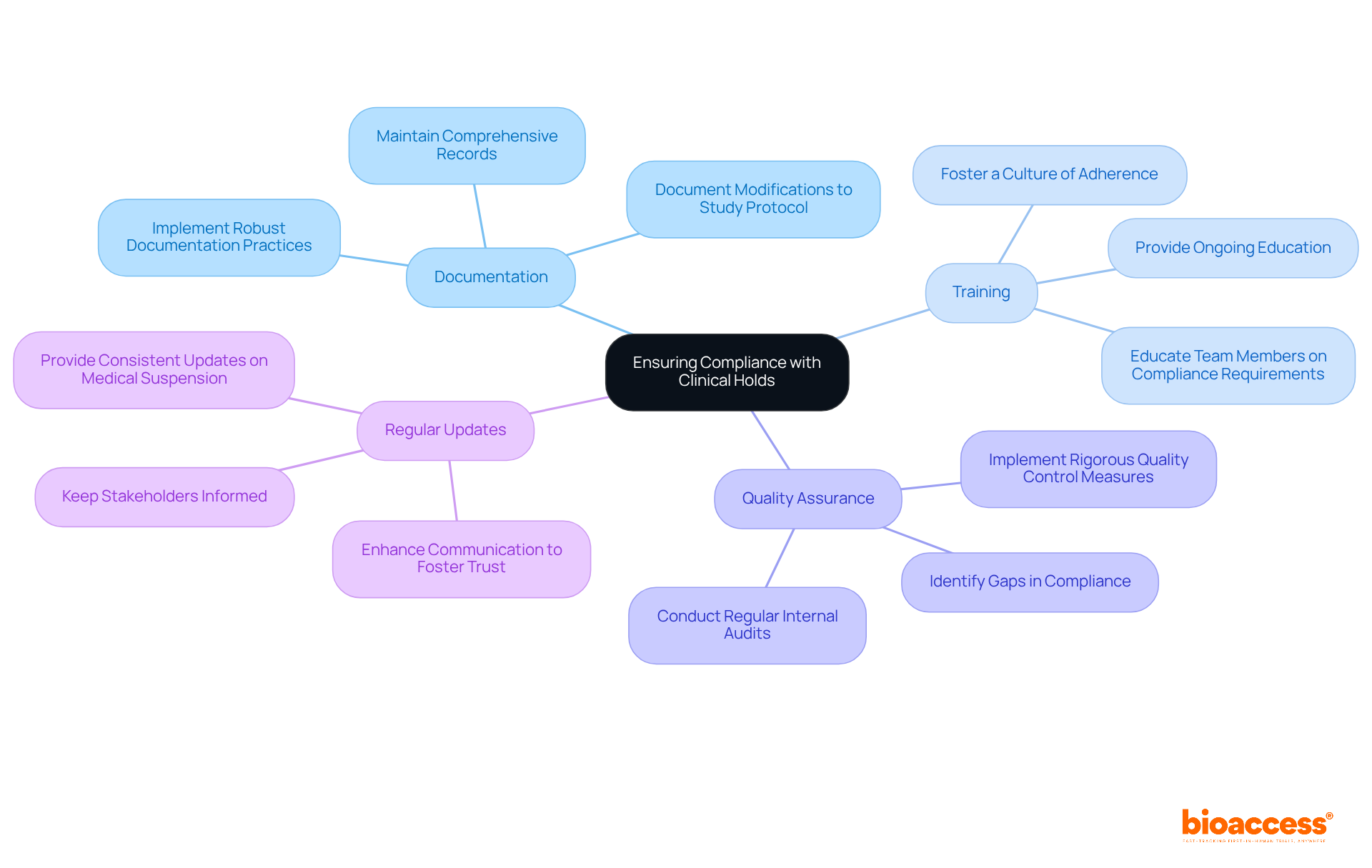
Ensuring compliance during a clinical hold is crucial for sponsors. To effectively manage this challenge, consider these key strategies:
Documentation: Maintain comprehensive records of all communications with the FDA, including any modifications to the study protocol. Effective documentation practices are essential; studies show that organizations with robust documentation processes experience significantly fewer compliance issues.
Training: Ensure that all team members are well-versed in compliance requirements and understand the implications of the research hold. Ongoing education fosters a culture of adherence, which is vital for navigating the complexities of research trials.
Quality Assurance: Implement rigorous quality control measures to ensure that all elements of the research comply with established standards. Regular internal audits can help identify potential gaps in compliance, allowing for timely corrective actions.
Regular Updates: Keep stakeholders informed with consistent updates regarding the status of the medical suspension and the actions being taken to resolve it. Openness in communication not only fosters trust but also enhances the overall compliance stance for future studies.
By prioritizing these compliance strategies, sponsors can effectively navigate the challenges posed by clinical holds while reinforcing their commitment to regulatory excellence.
The role of the sponsor investigator in holding the IND is pivotal in the landscape of clinical research, particularly in accelerating the journey of innovative therapies to market. Understanding the complexities of IND applications and potential challenges, such as clinical holds, enables sponsors to navigate the regulatory environment effectively, ensuring that their studies are both compliant and efficient.
Key insights from the article emphasize the importance of:
It outlines critical reasons for clinical holds and provides a structured approach for managing these situations effectively. By prioritizing documentation, training, and quality assurance, sponsors can significantly enhance their chances of overcoming regulatory obstacles and advancing their clinical trials.
Ultimately, the significance of these insights extends beyond individual studies; they underscore the broader imperative for agility and diligence in clinical research. Stakeholders are encouraged to adopt proactive strategies and leverage expert partnerships to ensure that groundbreaking therapies reach those in need without unnecessary delays. Embracing these practices not only fosters innovation but also strengthens the overall integrity of clinical research in the ever-evolving healthcare landscape.
What is bioaccess® and what does it specialize in?
bioaccess® is a company that specializes in simplifying the IND (Investigational New Drug) application process, particularly focusing on early-phase studies.
How quickly can bioaccess® secure ethical approvals for IND applications?
bioaccess® can secure ethical approvals in an impressive 4-6 weeks, which is significantly faster than traditional timelines.
Why is the rapid turnaround of ethical approvals important for sponsors?
The rapid turnaround is crucial for sponsors who are eager to initiate trials when the sponsor investigator holds the IND, thereby accelerating the journey to market for innovative therapies.
In which regions does bioaccess® operate?
bioaccess® operates in Latin America, the Balkans, and Australia.
What types of organizations does bioaccess® partner with?
bioaccess® partners with Medtech, Biopharma, and Radiopharma innovators.
What recent trends highlight the importance of swift ethical approvals in clinical research?
Recent advancements in IND applications underscore the growing importance of swift ethical approvals, especially when the sponsor investigator holds the IND, as organizations recognize the need for agility in research to meet evolving market demands.
How does collaboration with bioaccess® benefit stakeholders in clinical research?
Collaboration with bioaccess® helps stakeholders navigate the complexities of clinical research more effectively, ensuring that groundbreaking therapies reach those who need them most.