

The article delineates four essential practices for effective formulations within the pharmaceutical industry, emphasizing the critical importance of:
Each practice is bolstered by examples that showcase substantial enhancements in efficiency, accuracy, and financial performance. These illustrations serve to demonstrate how these strategies are pivotal in the successful development and management of pharmaceutical formulations.
In the rapidly changing pharmaceutical landscape, the effectiveness of formulations is fundamentally rooted in robust practices that ensure data integrity and connectivity. By implementing strategic approaches such as centralized data repositories and competitive intelligence, organizations can unlock significant efficiencies and drive innovation. Yet, a pressing challenge persists: how can pharmaceutical companies adeptly navigate the complexities of data management while maximizing their return on investment? This article delves into four essential practices that not only enhance operational effectiveness but also future-proof the pharmaceutical formulation process.
To ensure information integrity in clinical research, pharmaceutical firms must establish comprehensive management frameworks that include regular audits and validation procedures. The implementation of electronic data capture (EDC) tools not only enhances accuracy but also facilitates real-time monitoring. Moreover, it is essential to educate personnel on the principles of information integrity and the importance of precise documentation.
For example, a leading biopharma company adopted an EDC system, resulting in a 30% reduction in data entry errors, thereby significantly improving the reliability of their clinical trial data.
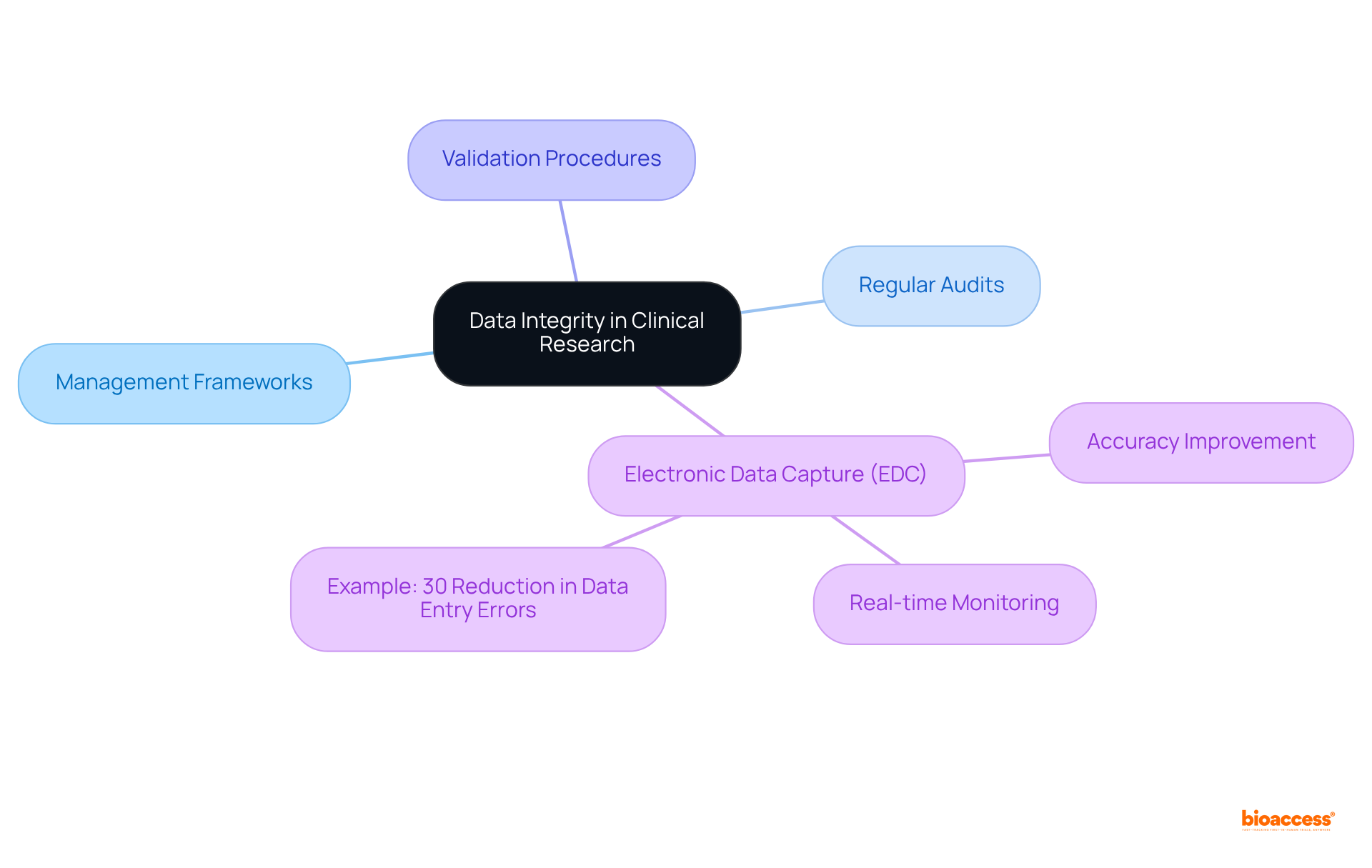
To enhance information organization, organizations must establish a centralized repository accessible to all teams. Implementing a common information model, such as the OMOP Common Information Model (CIM), significantly improves the integration of information from diverse sources, streamlining workflows and minimizing discrepancies.
Regular workshops and training sessions are essential to instill the importance of consistency among team members, especially in the context of generative AI and information management.
For instance, a pharmaceutical firm that adopted a standardized information structure experienced a remarkable 40% increase in project efficiency, enabling teams to exchange and analyze formulations in pharma seamlessly, thereby reducing confusion and fostering collaboration.
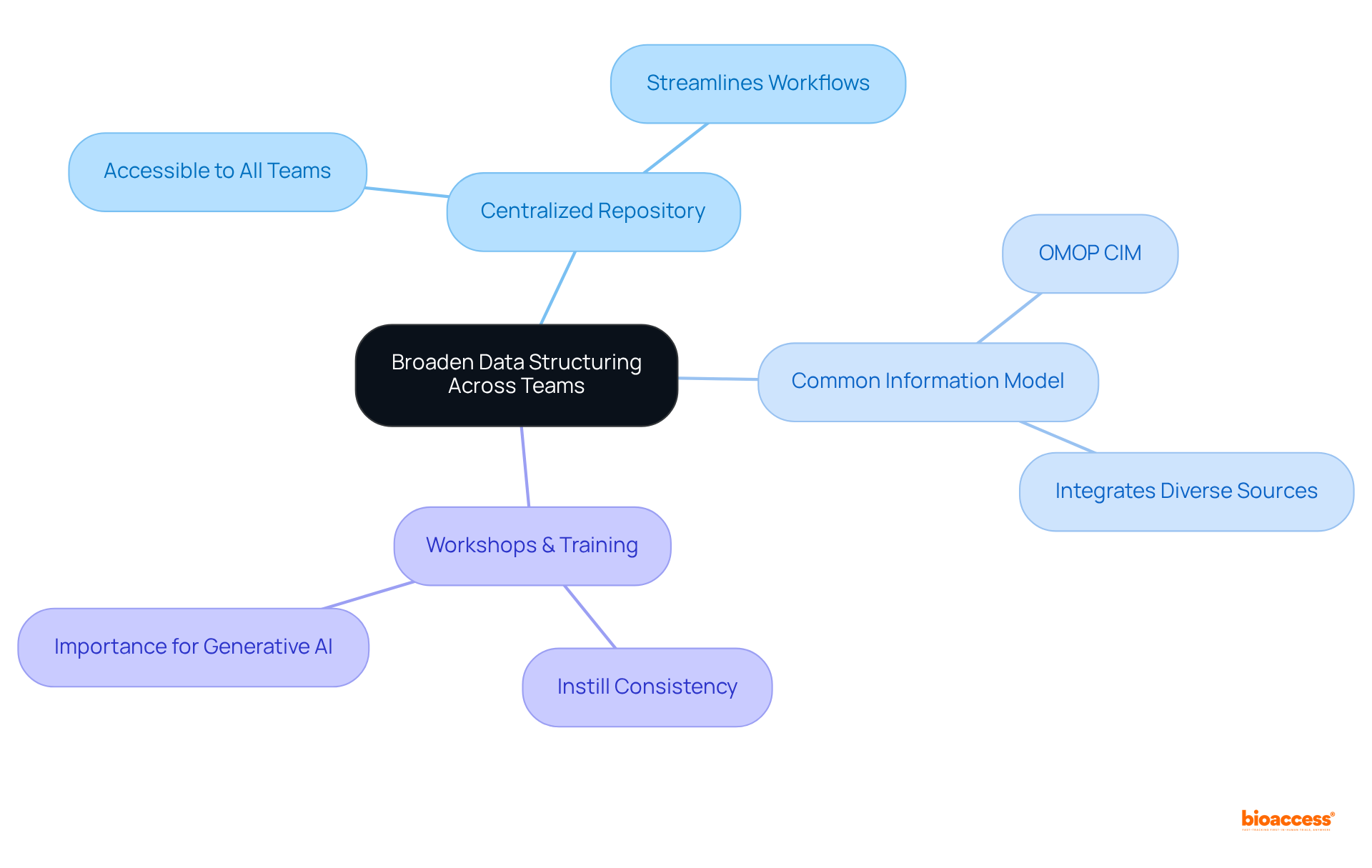
To enhance ROI through competitive intelligence, companies must invest in analytics tools that deliver actionable insights into market trends and rival strategies. Regular market assessments and competitor analyses are essential for identifying gaps and opportunities.
For instance, bioaccess® provides ethical approvals in just 4-6 weeks and achieves enrollment rates that are 50% faster than traditional markets, enabling quicker advancements from first-in-human studies to commercialization. A biopharma firm that adopted a competitive intelligence platform successfully pinpointed a niche market for a new drug, resulting in a remarkable 25% increase in sales within the first year of its launch.
As Massimo Orlando states, "Through Cortellis Competitive Intelligence, we have the most reliable and updated source of drug intelligence to drive our Marketing and Business Development strategic choices." This illustrates how utilizing analytics not only improves strategic decision-making but also greatly enhances financial performance related to formulations in pharma.
Engaging Key Opinion Leaders (KOLs) further amplifies the effectiveness of competitive intelligence, providing insights that shape perceptions and accelerate product adoption.
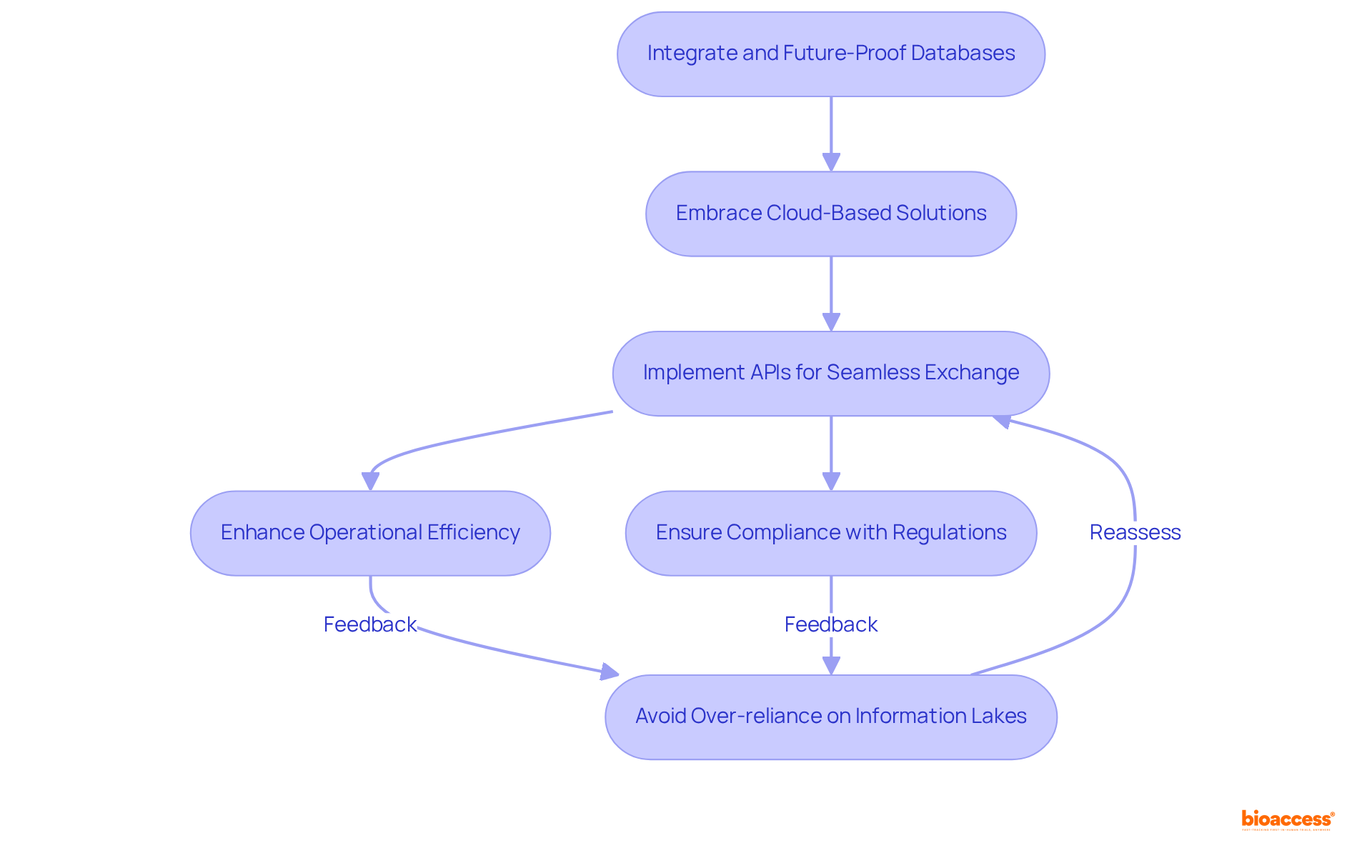
To effectively integrate and future-proof databases, organizations must embrace cloud-based solutions that offer both scalability and flexibility. The implementation of application programming interfaces (APIs) is essential for enabling seamless information exchange between diverse systems. APIs contribute to approximately one-third of all revenue for companies, underscoring their financial significance and relevance within formulations in pharma. By facilitating real-time information access and interoperability, APIs enhance operational efficiency and support adherence to regulatory standards.
Moreover, the quality and integration of information are crucial for precision medicine and AI in the biopharma sector, as they significantly enhance the development of formulations in pharma, enabling more accurate patient targeting and treatment personalization. For instance, a pharmaceutical firm that transitioned to a cloud-based information system not only improved accessibility but also achieved a 20% reduction in operational expenses while ensuring compliance with the latest data protection regulations for formulations in pharma. However, organizations must remain vigilant against common pitfalls, such as over-reliance on information lakes, which can result in a lack of standardization and impede effective integration.
This strategic approach illustrates the transformative impact of APIs on information integration in clinical research, empowering biopharma companies to streamline processes and enhance collaboration across platforms, which is critical for developing effective formulations in pharma. Establishing relationships among different data types is also vital for effective integration, ensuring that data can be utilized to its fullest potential.
Implementing effective practices in pharmaceutical formulations is essential for enhancing data integrity, streamlining processes, and maximizing return on investment. By focusing on robust management frameworks, organizations can ensure that their clinical research data is accurate and reliable, ultimately leading to better decision-making and improved outcomes in drug development.
This article highlights four key practices:
Each practice not only addresses specific challenges but also contributes to a more cohesive and efficient approach to pharmaceutical formulations. Real-world examples demonstrate significant improvements in project efficiency and financial performance, showcasing the tangible benefits of these strategies.
As the pharmaceutical industry continues to evolve, embracing these best practices will be crucial for staying competitive and meeting the demands of modern healthcare. Organizations are encouraged to invest in the right tools and training to foster collaboration and innovation, ensuring that they are well-equipped to navigate the complexities of drug development and deliver effective solutions to patients.
What is the importance of data integrity in clinical research?
Data integrity is crucial in clinical research as it ensures the accuracy and reliability of the information collected, which is essential for the validity of clinical trial results.
How can pharmaceutical firms ensure data integrity?
Pharmaceutical firms can ensure data integrity by establishing comprehensive management frameworks that include regular audits and validation procedures.
What role do electronic data capture (EDC) tools play in clinical research?
EDC tools enhance accuracy and facilitate real-time monitoring of data, which contributes to improved data integrity in clinical trials.
What are some benefits of implementing an EDC system?
Implementing an EDC system can lead to significant improvements, such as a reduction in data entry errors; for instance, one biopharma company reported a 30% reduction in errors after adopting an EDC system.
Why is personnel education important in maintaining data integrity?
Educating personnel on the principles of information integrity and the importance of precise documentation is essential to ensure that everyone involved understands their role in maintaining accurate and reliable data.