


When selecting a customs clearance partner for medical devices in Mexico, companies must prioritize the following criteria:
These criteria are critical, as a capable partner not only ensures compliance with local regulations but also enhances market entry strategies. Given the complexities of the medical device sector in Mexico, this partnership is essential for navigating challenges effectively.
Navigating the intricate landscape of customs clearance for medical devices in Mexico demands a strategic approach, given the stringent regulations and a rapidly evolving market. Companies aiming to enter this lucrative sector must grasp the critical role a customs clearance partner plays in ensuring compliance and efficiency. Yet, with a multitude of options available, how can businesses discern the right partner to facilitate seamless market entry while adhering to local laws?
This guide outlines essential steps and criteria for selecting a customs clearance partner, empowering organizations to make informed decisions that will enhance their operational success in Mexico's healthcare equipment sector.
Having a customs clearance partner in Mexico for medical devices represents a complex undertaking that necessitates strict compliance with local regulations. The initial step involves obtaining the necessary import permits, which must be requested electronically through the Digital International Trade Single Window (VUCEM). These permits typically require several weeks to process and are valid for 180 days or until the authorized amount has passed through inspection.
Understanding the Harmonized System (HS) tariff codes is essential, as these codes dictate the specific customs regulations relevant to each healthcare product, especially when working with a customs clearance partner Mexico medical devices. Furthermore, grasping the regulations established by COFEPRIS (the Federal Commission for the Protection Against Sanitary Risks) is crucial for our customs clearance partner in Mexico for medical devices, as these guidelines ensure the safety and effectiveness of imported healthcare products.
In 2025, collaborating with a customs clearance partner Mexico medical devices will be vital for maneuvering through these regulatory frameworks efficiently to achieve successful market entry and compliance in Mexico's rapidly expanding healthcare equipment sector. Notably, the revenue of the healthcare equipment market in Mexico reached $8 billion in 2023, underscoring its significance and growth potential. As Jennifer Mendoza remarked, 'Mexico is among the primary exporters of healthcare technology in the world,' highlighting the nation's prominent position in the global healthcare technology arena.
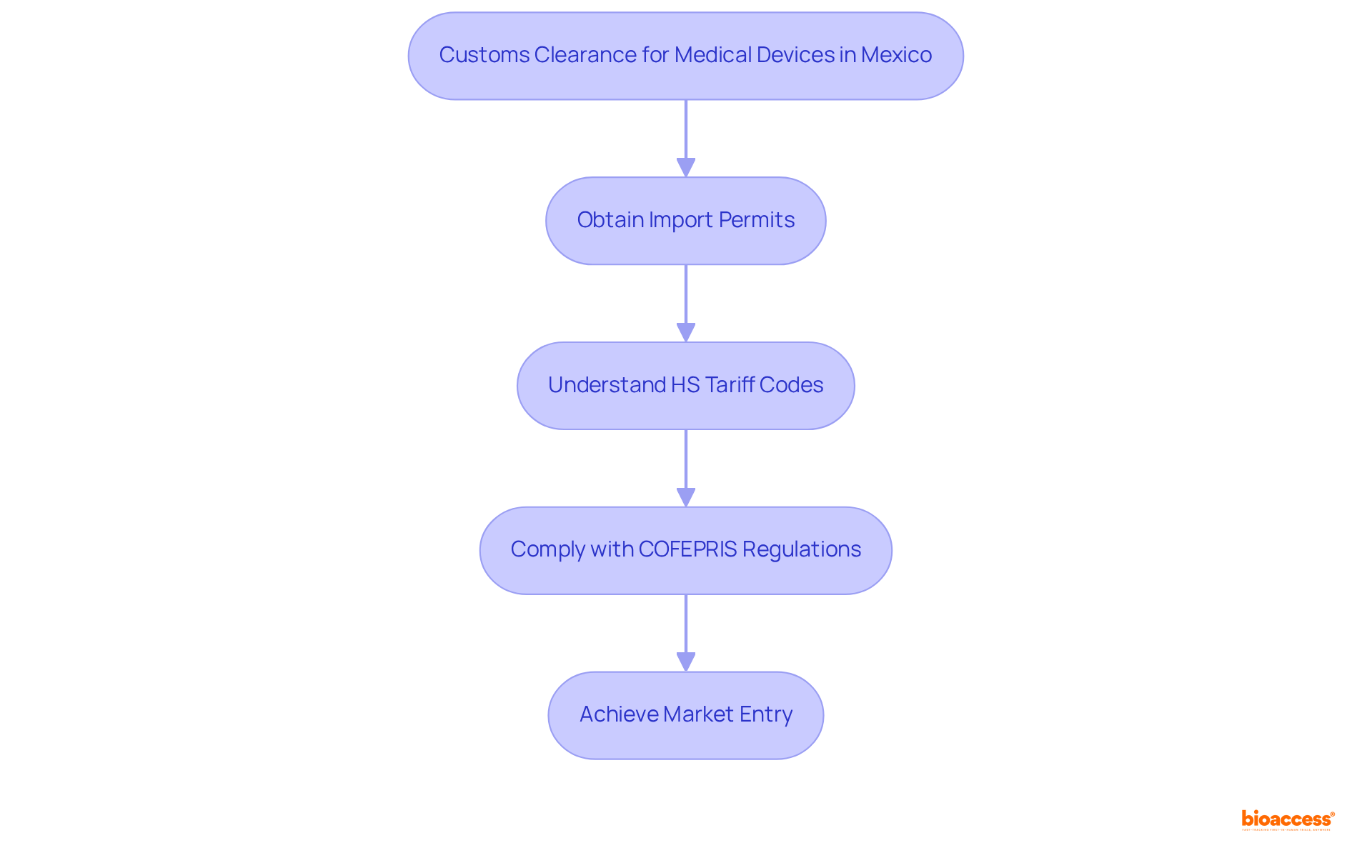
When choosing a customs clearance partner for Mexico medical devices, it is imperative to prioritize several key criteria to ensure compliance and efficiency.
Experience in Medical Device Imports: Choosing a partner with a proven track record in managing medical device imports is essential. This sector is governed by specific guidelines, and a thorough understanding of these requirements can significantly influence the success of the import procedure.
Regulatory Knowledge: A comprehensive understanding of COFEPRIS regulations and the customs clearance process in Mexico is crucial. Partners must adeptly navigate intricate regulatory environments to guarantee that all healthcare products meet safety and compliance standards before entering the market. As industry experts highlight, "By teaming with an experienced IOR partner, businesses can save time, reduce costs, and focus on providing life-saving medical devices to those in need."
Technology Utilization: Opting for partners that leverage advanced technology for tracking shipments and managing documentation is advisable. This capability can streamline the clearance procedure, mitigate delays, and enhance overall operational efficiency.
Reputation and References: Investigating the partner's standing within the industry is vital. Gathering references from other clients can provide insights into their reliability and effectiveness, which are crucial for maintaining compliance and ensuring timely delivery.
Cost Transparency: Clear communication regarding fees and potential additional costs is essential. A transparent pricing system aids in preventing unforeseen costs during the clearance process, facilitating improved budget management.
By concentrating on these standards, companies can forge effective alliances with a customs clearance partner in Mexico for medical devices, ensuring adherence to local regulations and enhancing market entry strategies. The Clearance Service Market, valued at USD 25.57 Billion in 2023 and projected to reach USD 44.68 Billion by 2031, underscores the significance of selecting a capable clearance partner.
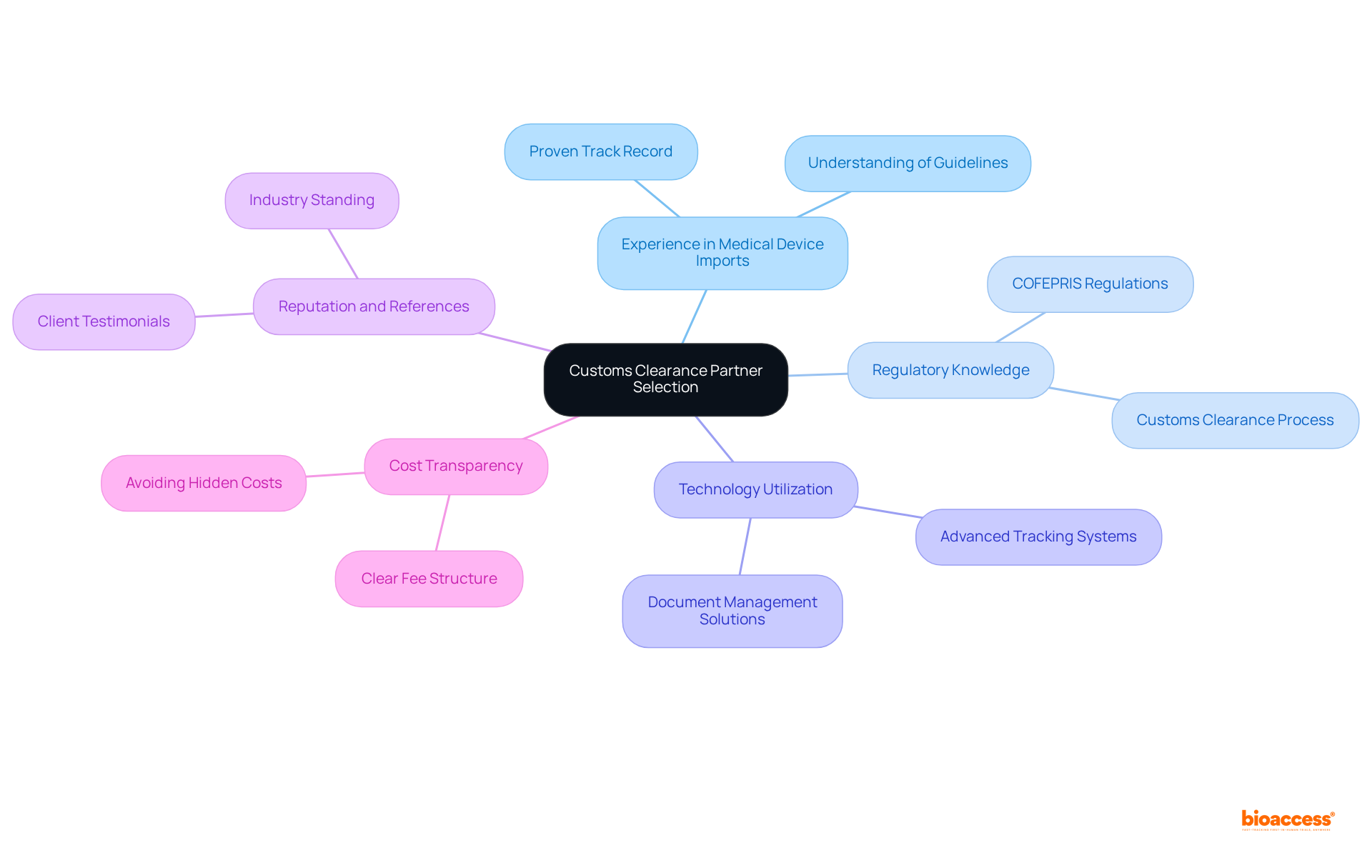
To effectively evaluate and vet potential customs clearance partners for medical devices in Mexico, it is essential to consider the following steps:
Conduct Interviews: Arrange conversations with prospective partners to investigate their experience, procedures, and knowledge of health equipment regulations. This dialogue is crucial for assessing their expertise and alignment with your needs.
Request Documentation: Obtain documentation that showcases their compliance history, including relevant certifications and licenses. This information is vital for ensuring they meet the regulatory standards required for medical device imports.
Check References: Reach out to previous clients to gather insights about their experiences with the partner. Focus on aspects such as reliability, communication, and problem-solving capabilities, as these factors are essential for a successful partnership.
Assess Technology Capabilities: Evaluate the technology tools used by the partner for tracking shipments and handling documentation. Advanced technology can greatly improve efficiency and precision in border control operations.
Review Performance Metrics: Request data on their performance metrics, including clearance times and error rates. Examining these metrics will assist in assessing their efficiency in handling border processes and ensuring prompt delivery of medical devices.
In the framework of the expanding Mexico Brokerage Market, expected to attain USD 345.65 million by 2025, choosing the right customs clearance partner for Mexico medical devices is increasingly vital. As Dylan Sommer highlights, "If you wish to remain knowledgeable about changing regulations and trade policies, it’s crucial that you allocate time to routinely check news releases, official updates, and publications from government agencies and industry news outlets."
By adhering to these steps and taking into account the wider market context, you can guarantee a comprehensive evaluation that aligns with the regulatory framework and operational requirements of the medical device sector in Mexico.
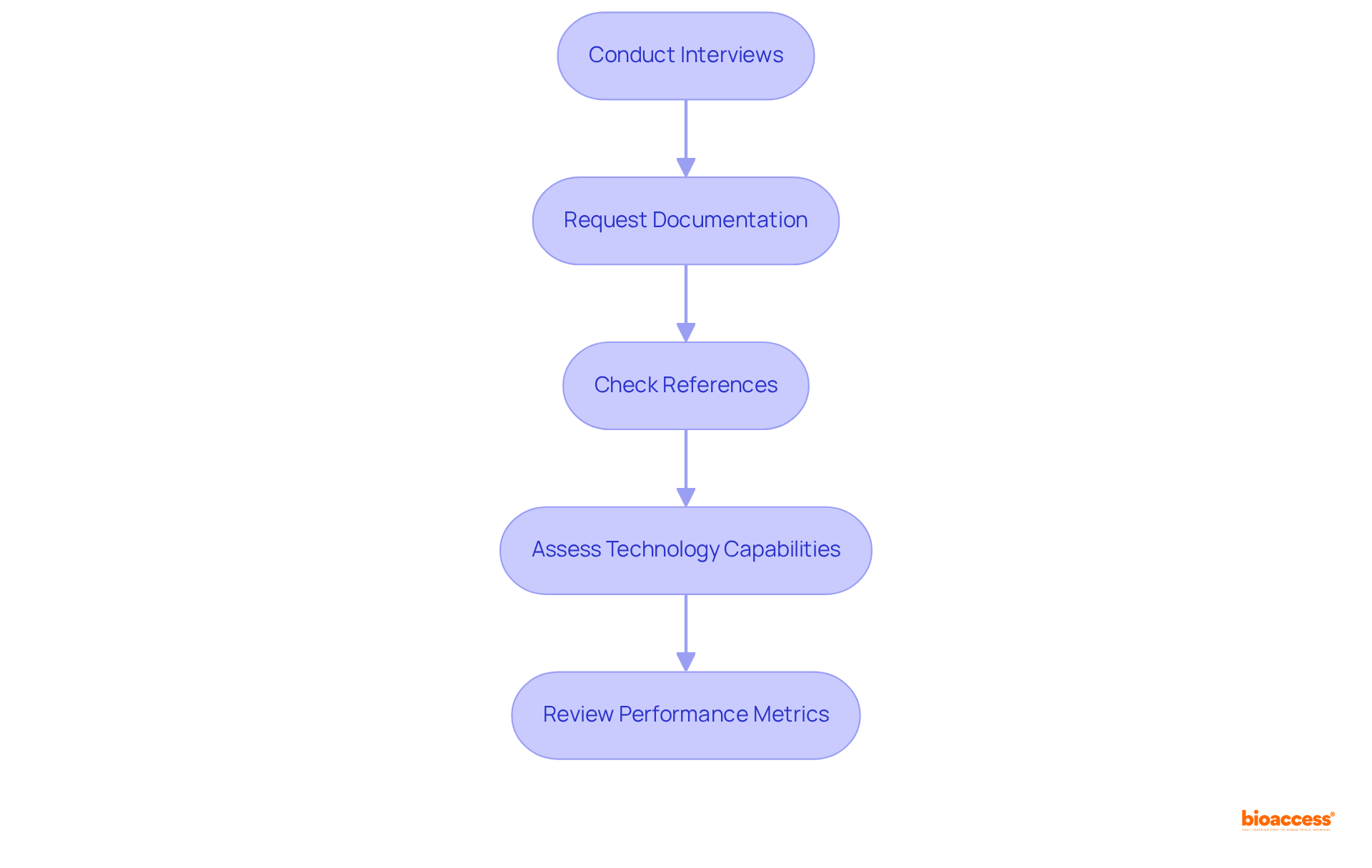
To cultivate a successful partnership with your customs clearance partner Mexico medical devices, it is essential to implement strategic measures that ensure both effectiveness and efficiency.
Set Clear Expectations: Articulate your expectations regarding timelines, documentation, and compliance requirements. This clarity ensures that both parties are aligned from the outset, facilitating a smoother process.
Maintain Open Communication: Foster an atmosphere that encourages open dialogue. This enables both parties to address challenges and exchange updates on procedures, which is crucial for prompt resolutions.
Regular Meetings: Schedule consistent meetings to evaluate performance, address any issues, and discuss upcoming shipments or regulatory changes. Regular interactions can significantly enhance the efficiency of a customs clearance partner Mexico medical devices, as evidenced by improved turnaround times in organizations that prioritize communication. For instance, enhancing operational efficiency can reduce documentation turnaround times by as much as 40%.
Feedback Mechanism: Establish a feedback system to facilitate continuous improvement in the partnership. This allows both parties to learn from past experiences and refine processes for better outcomes.
Joint Problem-Solving: Approach challenges collaboratively, tackling them as a unified team. This not only fosters a sense of collaboration but also ensures adherence to trade regulations, ultimately benefiting both parties. As Michael Jordan famously stated, "Talent wins games, but teamwork and intelligence win championships," underscoring the significance of collaboration in achieving successful customs clearance.
Navigating the complexities of customs clearance for medical devices in Mexico demands a strategic approach and the right partner. Selecting a customs clearance partner is not merely a logistical decision; it is a crucial factor that can determine the success of market entry and compliance in a rapidly evolving healthcare landscape. With Mexico’s healthcare equipment market exhibiting significant growth potential, aligning with an experienced and knowledgeable partner is essential for ensuring adherence to regulatory standards and facilitating smooth operations.
This article highlights several key considerations when choosing a customs clearance partner, including:
It underscores the importance of evaluating potential partners through interviews, documentation requests, and performance metrics, ensuring they possess the qualifications necessary to navigate the regulatory landscape effectively. Moreover, establishing a collaborative relationship through clear expectations and open communication fosters an environment conducive to resolving challenges and enhancing operational efficiency.
In conclusion, the significance of selecting the right customs clearance partner in Mexico cannot be overstated. As the healthcare equipment market continues to expand, businesses must prioritize strategic partnerships that facilitate compliance and operational success. By investing time and resources in finding a qualified partner, companies can not only streamline their customs clearance processes but also position themselves for long-term success in the competitive medical device sector.
What is the first step in customs clearance for medical devices in Mexico?
The first step involves obtaining the necessary import permits, which must be requested electronically through the Digital International Trade Single Window (VUCEM).
How long does it typically take to process import permits for medical devices in Mexico?
Import permits typically require several weeks to process.
How long are the import permits valid for medical devices in Mexico?
Import permits are valid for 180 days or until the authorized amount has passed through inspection.
Why is understanding Harmonized System (HS) tariff codes important?
Understanding HS tariff codes is essential because these codes dictate the specific customs regulations relevant to each healthcare product.
What organization’s regulations must be understood for customs clearance of medical devices in Mexico?
The regulations established by COFEPRIS (the Federal Commission for the Protection Against Sanitary Risks) must be understood.
Why are COFEPRIS regulations important for customs clearance?
COFEPRIS regulations ensure the safety and effectiveness of imported healthcare products.
What is the significance of collaborating with a customs clearance partner for medical devices in Mexico?
Collaborating with a customs clearance partner is vital for efficiently navigating regulatory frameworks to achieve successful market entry and compliance in Mexico's healthcare equipment sector.
What was the revenue of the healthcare equipment market in Mexico in 2023?
The revenue of the healthcare equipment market in Mexico reached $8 billion in 2023.
What position does Mexico hold in the global healthcare technology market?
Mexico is among the primary exporters of healthcare technology in the world, highlighting its prominent position in the global healthcare technology arena.