


The article presents a systematic approach to defining excipients in pharmaceutical research, underscoring their pivotal roles as inactive substances that enhance drug stability and efficacy. It delineates four essential steps:
This comprehensive framework ensures the safety and effectiveness of pharmaceutical formulations, highlighting the critical need for precision in the development of these formulations.
Understanding the vital role of excipients in pharmaceuticals is crucial for anyone involved in drug formulation. These inactive substances are not merely fillers; they significantly enhance drug stability, bioavailability, and overall efficacy, making them essential components of successful medications.
However, as the landscape of pharmaceutical research evolves, challenges arise in defining and categorizing these excipients effectively. How can researchers navigate the complexities of excipient selection while ensuring compliance and maintaining high standards of quality?
This article outlines a comprehensive four-step approach to excipient definition, equipping professionals with the tools needed to overcome obstacles and optimize drug formulations.
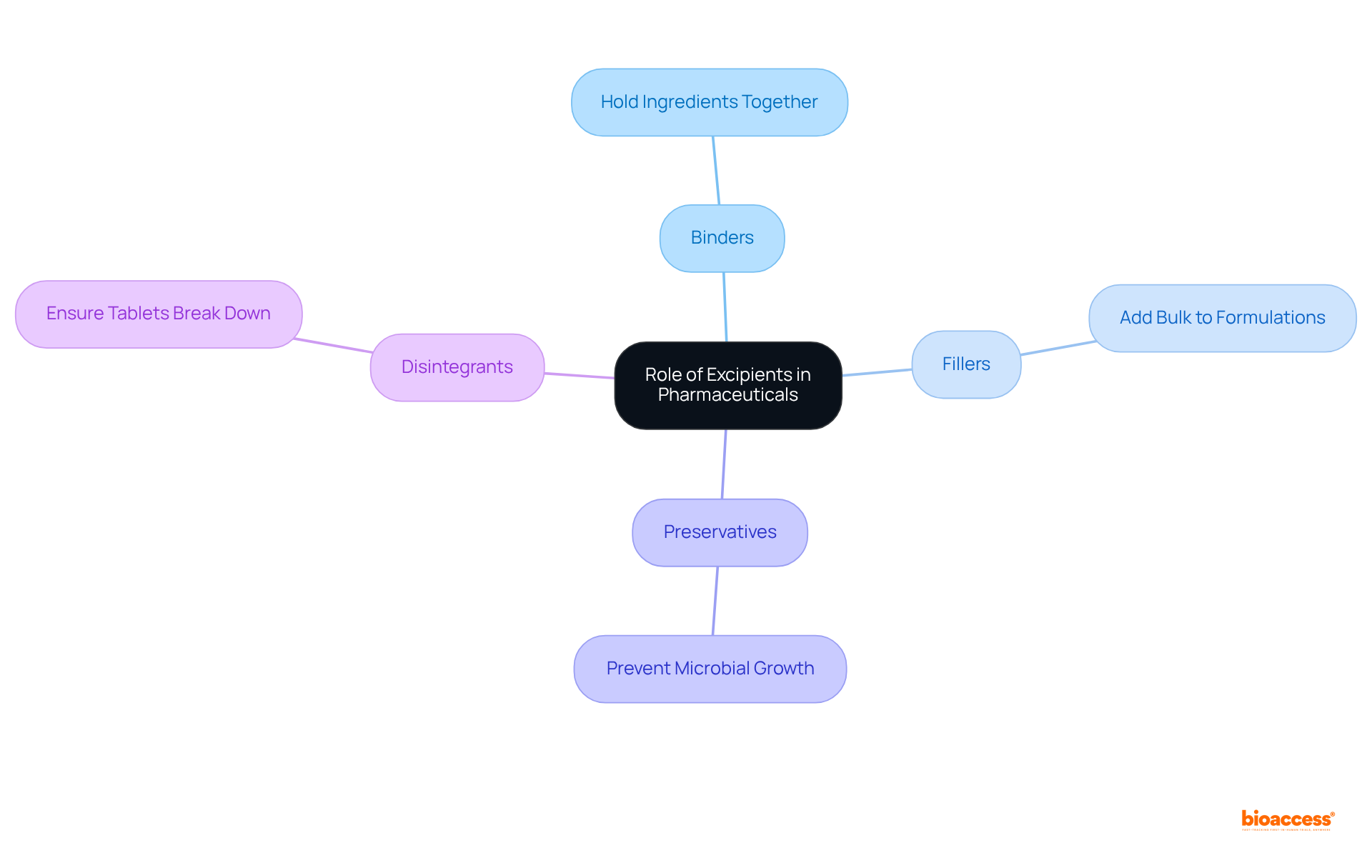
To excipient define, they are essential inactive substances that serve as carriers for the active ingredients in medications. Their roles are multifaceted, significantly enhancing drug stability, improving bioavailability, and streamlining the manufacturing process. Understanding the excipient define different categories—such as binders, fillers, preservatives, and disintegrants—is crucial for recognizing their roles in drug efficacy and safety.
For instance, binders are vital for holding ingredients together, while disintegrants ensure that tablets break down effectively in the body. Recent studies indicate that inactive ingredients constitute 80-90% of the final product, underscoring their significance in formulation. Moreover, additives can greatly influence the stability of medicinal products, impacting both shelf-life and effectiveness.
As the biologics market continues to expand, with an anticipated compound annual growth rate of 15% until 2027, the role of additives in enhancing active ingredient stability and bioavailability becomes increasingly essential. By acknowledging these functions, professionals can gain a deeper understanding of how additives contribute to the overall effectiveness and safety of pharmaceutical products.
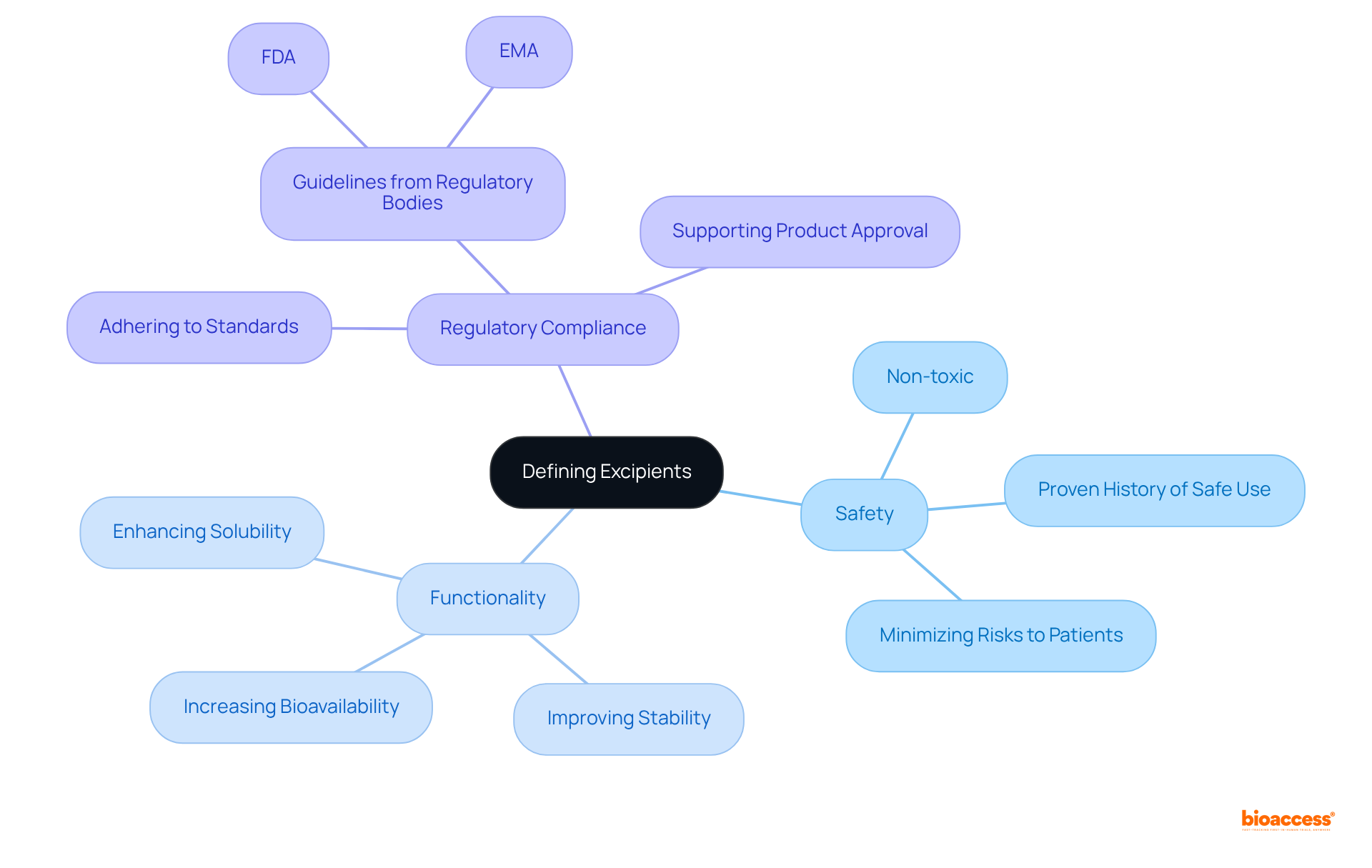
To effectively define excipients, it is essential to establish clear criteria encompassing safety, functionality, and adherence to regulations. Begin by consulting pharmacopoeias and guidelines from regulatory bodies such as the FDA and EMA. Consider the following key aspects:
Document these criteria clearly to provide a robust framework to guide your selection process, ultimately supporting the development of safe and effective pharmaceutical products. By utilizing bioaccess®'s global-first clinical agility, you can improve your strategy for ingredient selection and ensure adherence to industry standards.
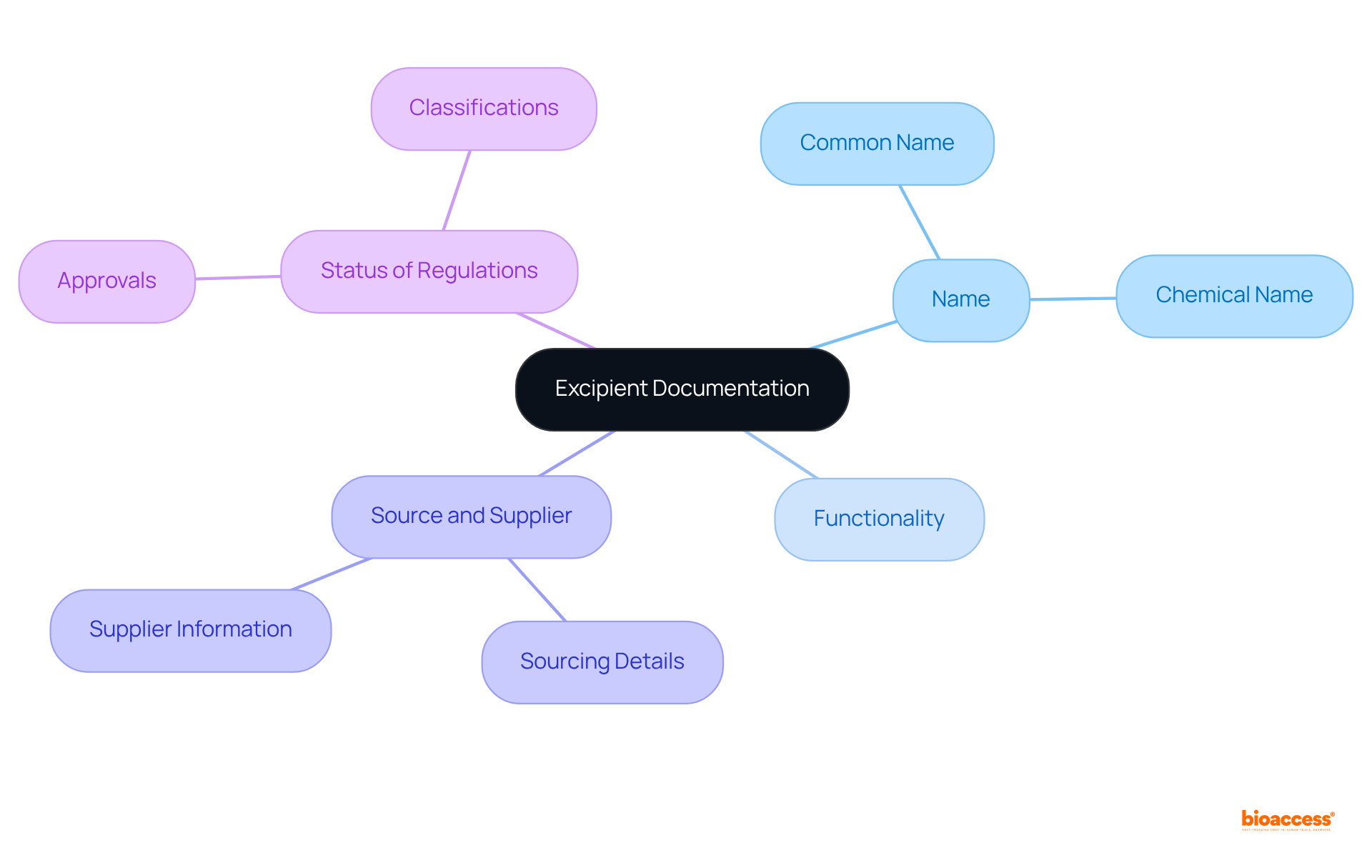
Establish a systematic database to excipient define and document their categorization. This can be accomplished through a spreadsheet or specialized software designed to track essential information:
This structured documentation not only streamlines the research process but also ensures compliance with legal requirements.
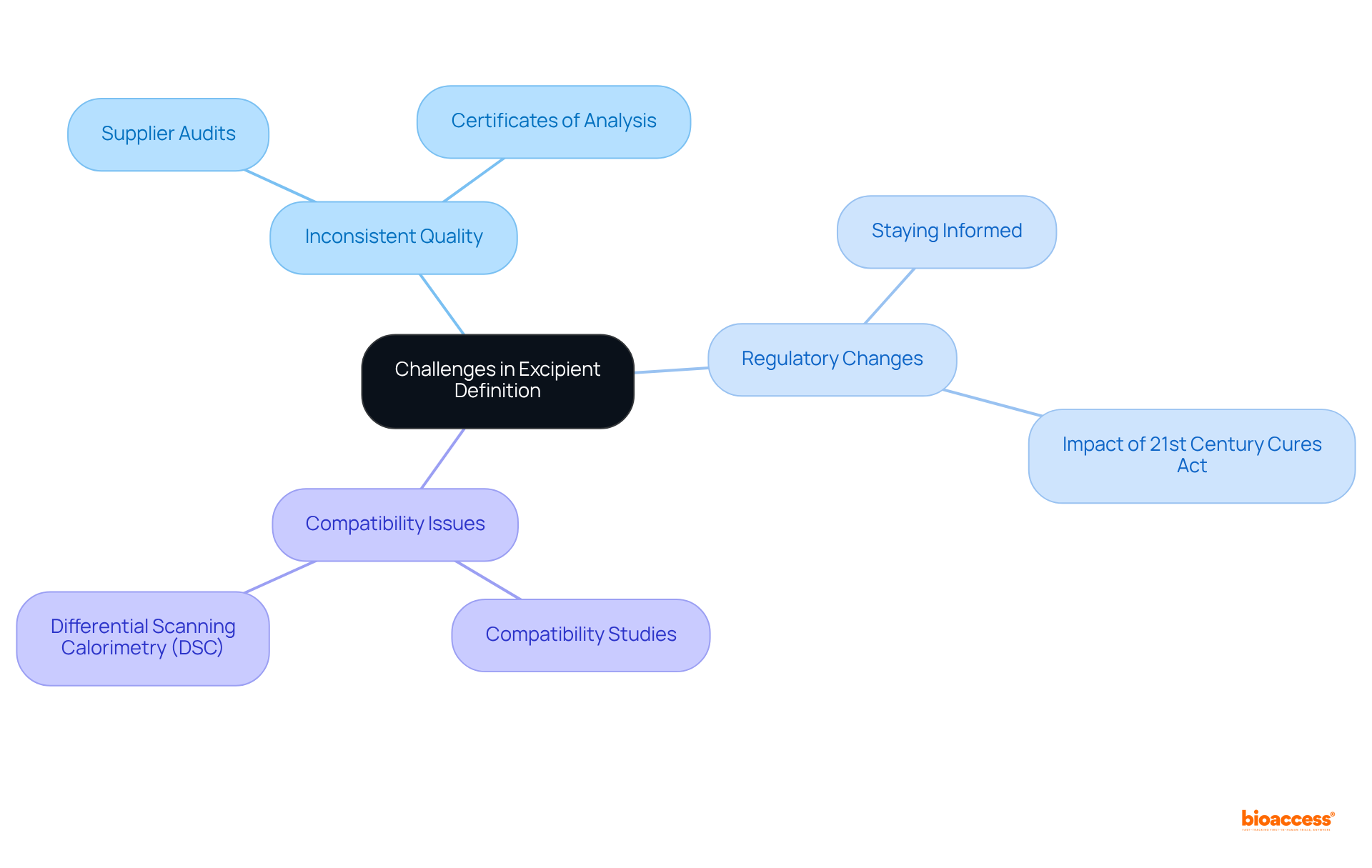
Defining excipient define in pharmaceutical research presents several challenges that require careful consideration.
Inconsistent Quality: It is crucial to ensure that suppliers deliver excipients that align with established quality criteria. Regular audits of suppliers and the procurement of certificates of analysis are essential practices to maintain high standards.
Regulatory Changes: The pharmaceutical landscape is continually evolving, with regulatory changes affecting the status and application of additives. Staying informed through industry newsletters and professional organizations can help navigate these shifts effectively. For example, U.S. government programs such as the 21st Century Cures Act have expedited drug development processes, indirectly aiding the ingredient market.
Compatibility Issues: Conducting thorough compatibility studies is vital to ascertain that inactive substances do not negatively interact with active ingredients. Techniques such as differential scanning calorimetry (DSC) can be employed to evaluate potential interactions.
By proactively tackling these challenges, researchers can maintain the integrity of ingredient definitions, ultimately resulting in successful outcomes in drug research. The pharmaceutical excipients market, valued at USD 9.5 billion in 2023, is projected to grow significantly to USD 17.8 billion by 2032, with a compound annual growth rate (CAGR) of 7.2% from 2024 to 2032. This growth highlights the significance of upholding high standards in ingredient quality and compliance. Additionally, stringent regulatory requirements for excipient define safety and compatibility pose challenges for manufacturers, as highlighted in the case study on 'Challenges of Regulatory Compliance.
Defining excipients in pharmaceutical research is a critical step in ensuring the efficacy and safety of drug formulations. By understanding the multifaceted roles that these inactive substances play, researchers can better appreciate their impact on drug stability, bioavailability, and overall product quality. This guide has outlined essential steps to effectively define excipients, emphasizing the importance of a structured approach that includes clear criteria, systematic documentation, and proactive problem-solving.
Key insights from the article highlight the necessity of establishing safety, functionality, and regulatory compliance as foundational criteria for excipient selection. Furthermore, the significance of systematic documentation cannot be overstated, as it not only streamlines the research process but also ensures adherence to legal standards. Addressing common challenges such as inconsistent quality, regulatory changes, and compatibility issues is essential for maintaining high standards in pharmaceutical development.
In light of the growing pharmaceutical excipients market and the evolving regulatory landscape, it is imperative for researchers and manufacturers to prioritize the definition and selection of excipients. By doing so, they can enhance drug delivery systems and improve patient outcomes. Embracing these strategies will not only contribute to successful drug formulation but also foster innovation in the pharmaceutical industry, paving the way for safer and more effective therapeutic solutions.
What are excipients in pharmaceuticals?
Excipients are essential inactive substances that serve as carriers for the active ingredients in medications.
What roles do excipients play in pharmaceuticals?
Excipients enhance drug stability, improve bioavailability, and streamline the manufacturing process.
What are the different categories of excipients?
The different categories of excipients include binders, fillers, preservatives, and disintegrants.
What is the function of binders in pharmaceuticals?
Binders are vital for holding ingredients together in a medication.
How do disintegrants function in drug formulations?
Disintegrants ensure that tablets break down effectively in the body.
What percentage of the final product do inactive ingredients constitute?
Inactive ingredients constitute 80-90% of the final product.
How do additives impact medicinal products?
Additives can greatly influence the stability of medicinal products, impacting both shelf-life and effectiveness.
What is the projected growth of the biologics market?
The biologics market is anticipated to have a compound annual growth rate of 15% until 2027.
Why is understanding the role of additives important for professionals in pharmaceuticals?
Understanding the role of additives is important as they contribute to the overall effectiveness and safety of pharmaceutical products.