


Navigating the landscape of clinical trials in Bulgaria presents a unique set of challenges and opportunities for researchers and sponsors alike. Stringent regulations, shaped by the EU Clinical Studies Regulation and local laws, make it essential to understand the intricacies of clinical trial agreements. This understanding is crucial for ensuring compliance and fostering successful outcomes.
This article delves into five essential practices that not only promote ethical research but also enhance the integrity and safety of clinical trials.
How can stakeholders effectively balance regulatory demands with the need for innovation and participant protection in this evolving field?
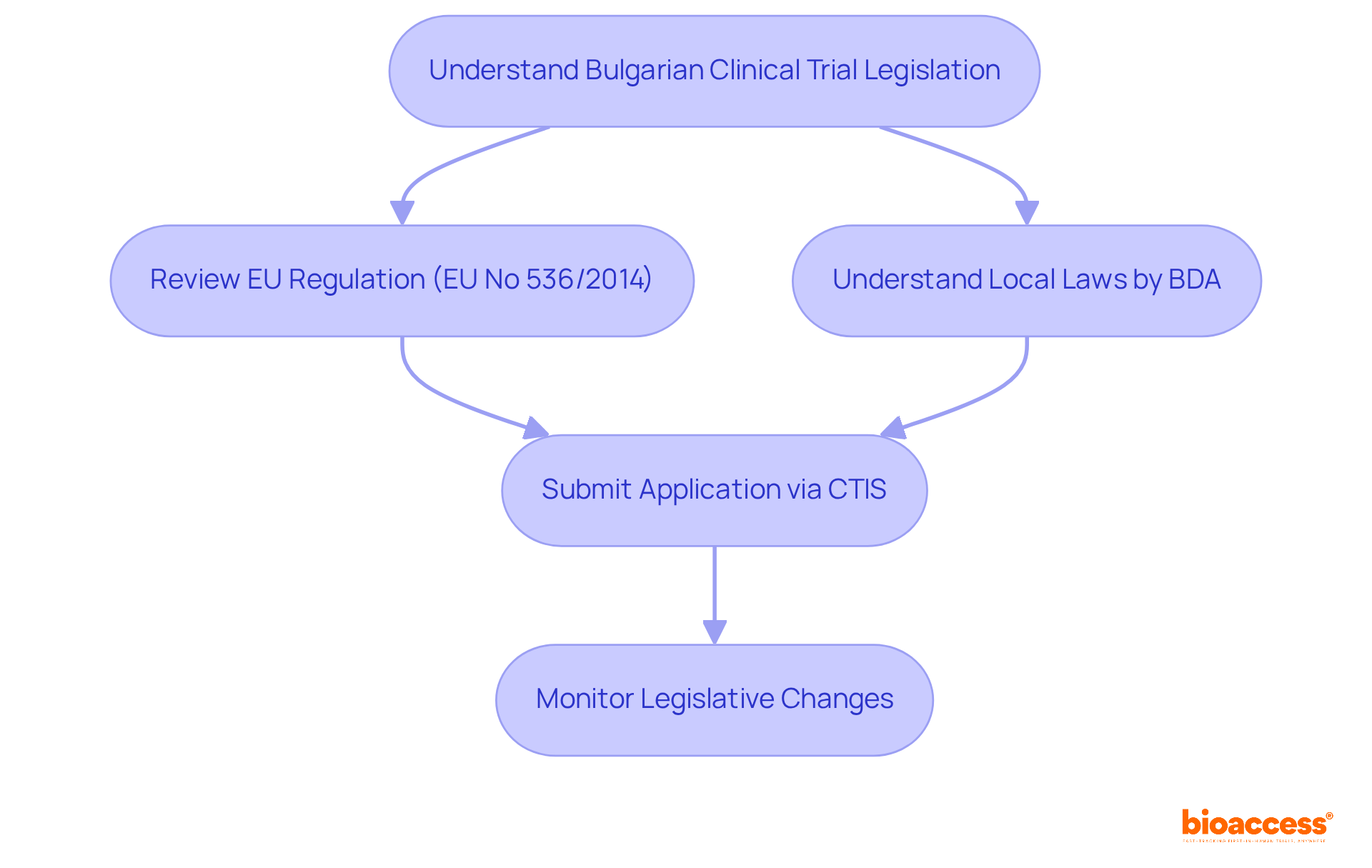
Conducting clinical studies in Bulgaria requires a solid grasp of the EU Clinical Studies Regulation (EU No 536/2014) and the local laws enforced by the Bulgarian Drug Agency (BDA). This regulation is pivotal, outlining the necessary requirements for testing approval, including submission methods and ethical considerations, which are essential for compliance with both EU and national standards. Sponsors must submit their applications through the Clinical Trials Information System (CTIS), a platform crafted to streamline the approval process and boost efficiency.
Moreover, staying informed about any changes to the legislation is vital, as these updates can significantly affect court timelines and requirements. The BDA's oversight guarantees that all medical trials meet high safety and quality standards, creating a trustworthy environment for innovative research. By understanding these regulations and processes, stakeholders can navigate the complexities of clinical trials more effectively.
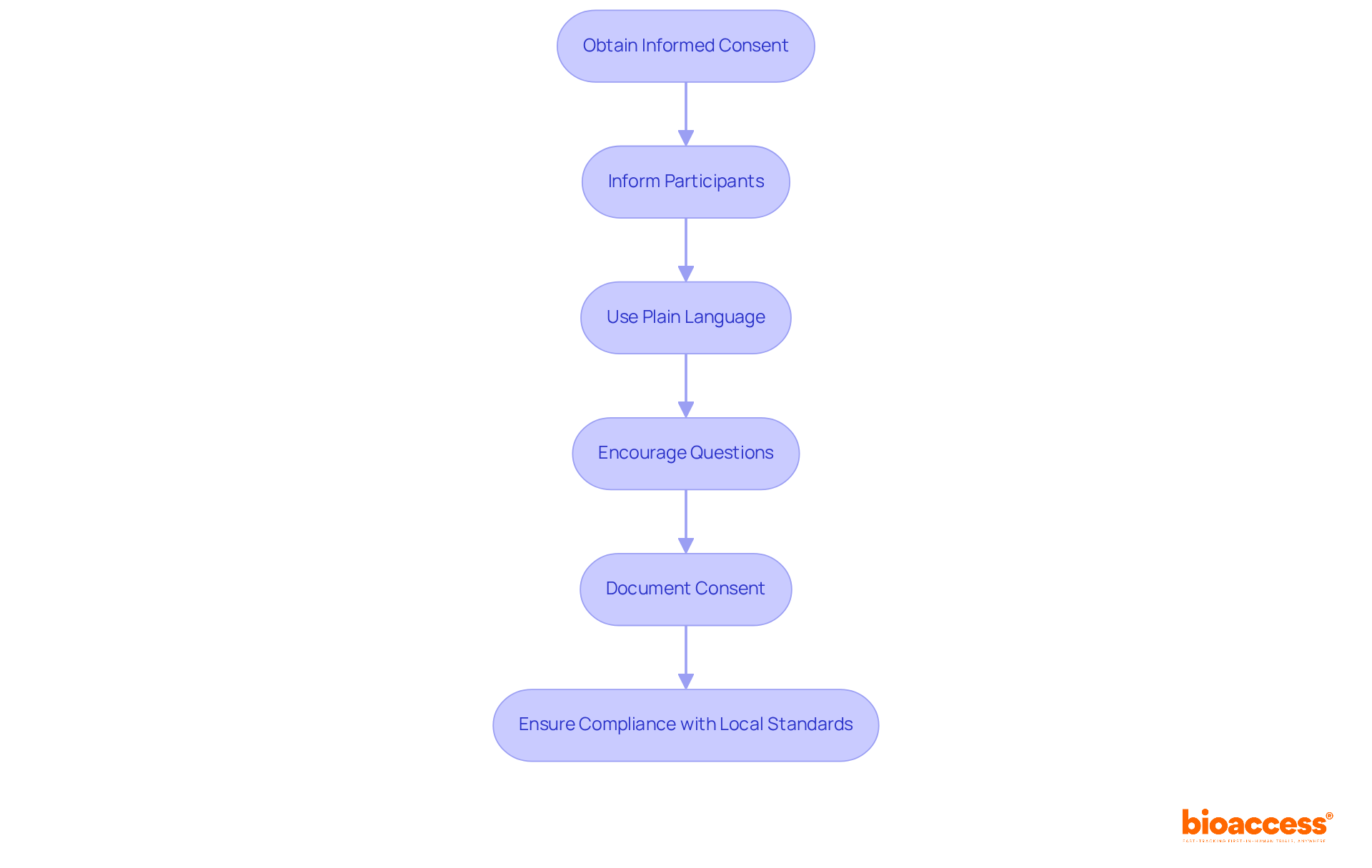
Informed consent stands as a cornerstone of ethical clinical research, ensuring that potential subjects are fully informed about the study's purpose, procedures, risks, and benefits. This practice is not just a regulatory requirement; it is essential for fostering trust and transparency in the research process. In Bulgaria, clinical trial agreements and contracts require that consent forms be drafted in Bulgarian and adhere to local regulatory standards, reinforcing the importance of compliance in clinical trials.
Researchers must prioritize clarity by employing plain language and steering clear of technical jargon. This approach significantly enhances understanding and empowers participants to make informed decisions. It is crucial to provide ample opportunities for attendees to ask questions, cultivating an environment of openness and confidence. Such engagement not only respects individuals' rights but also strengthens the ethical foundation of the research.
Moreover, meticulous documentation of the consent procedure serves dual purposes: it acts as legal protection and provides proof of adherence to ethical standards. This thorough method not only honors individuals' rights but also enhances the integrity of the research process. By committing to these practices, researchers can ensure that they uphold the highest ethical standards while advancing scientific knowledge.
A thorough research protocol is crucial for the successful implementation of clinical studies. It must clearly outline the study's objectives, design, methodology, and statistical analysis plan. In Bulgaria, the clinical trial agreements and contracts must also detail participant recruitment, data management, and safety monitoring.
To ensure compliance with national requirements, involving bioaccess in the feasibility and selection of research sites and principal investigators (PIs) is essential. Their expertise in assessing and providing input on study documents, along with overseeing setup and approvals from ethics committees and health ministries, significantly enhances the protocol development process. Regularly reviewing and updating the protocol as the study progresses is vital to address unforeseen challenges and maintain compliance with regulatory changes.

Choosing qualified investigators is essential in the clinical study process. Investigators must possess not only relevant medical and research experience but also a thorough understanding of Good Clinical Practice (GCP) guidelines. In Bulgaria, it is vital to ensure that investigators involved in clinical trial agreements and contracts in Bulgaria are registered with the appropriate regulatory bodies and have completed the necessary GCP training. This training is crucial, as it directly influences assessment outcomes by ensuring adherence to ethical standards and regulatory requirements.
Building a strong working relationship with investigators fosters effective communication and teamwork throughout the study. Additionally, providing ongoing training and support enhances investigators' capabilities to manage studies effectively and tackle any challenges that may arise. This ultimately contributes to the success of the research initiative.
In summary, the selection of qualified investigators is not just a procedural step; it is a foundational element that can significantly impact the outcomes of clinical research. By prioritizing their qualifications and fostering collaboration, we can navigate the complexities of clinical studies more effectively.

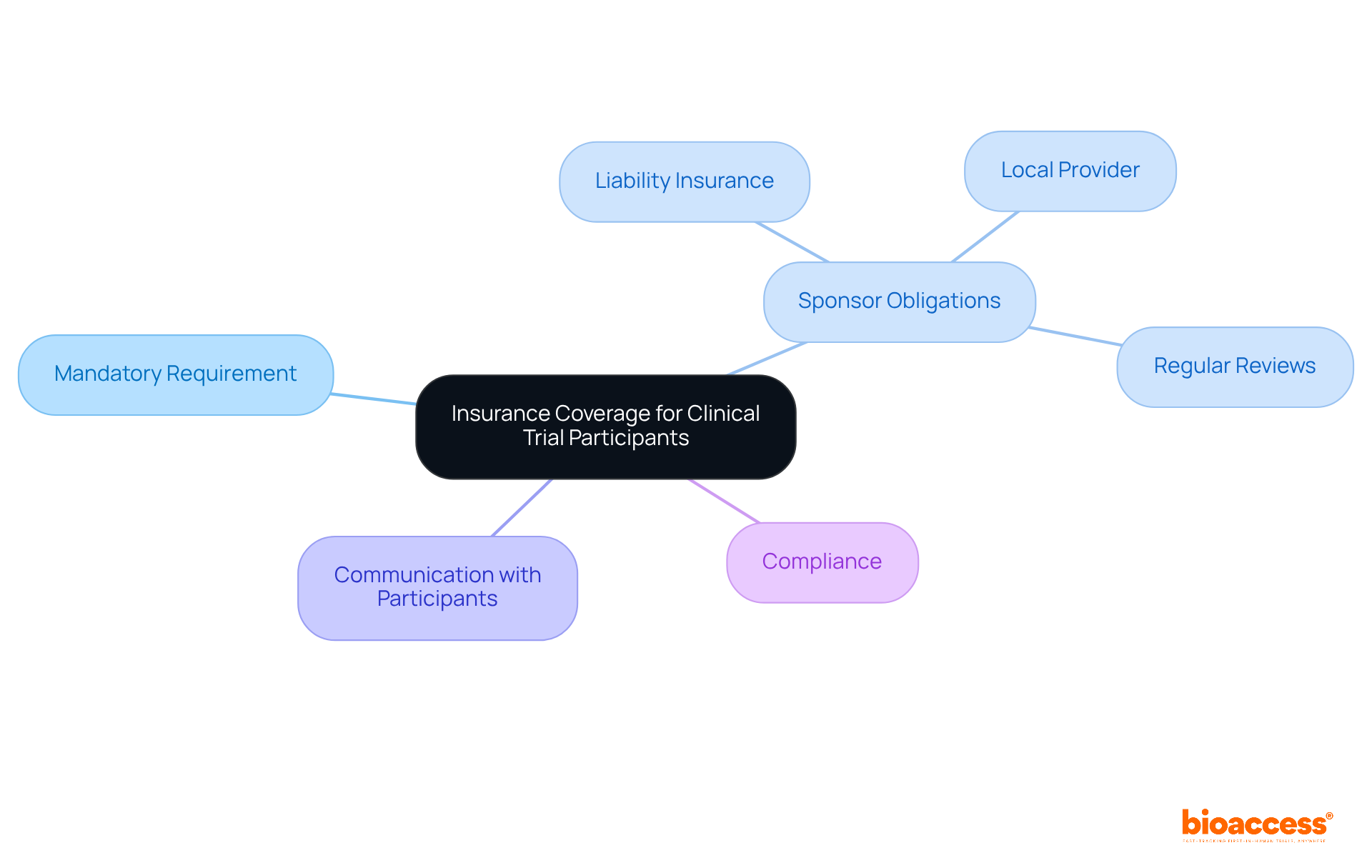
In Bulgaria, insurance coverage for individuals involved in clinical trials is not just a recommendation; it’s a mandatory requirement aimed at safeguarding participants from potential risks. Sponsors are obligated to secure liability insurance that comprehensively covers any material or non-material damages that may arise during the study. This insurance must be arranged through a locally licensed insurance provider to align with Bulgarian regulations.
It’s crucial to communicate the specifics of the insurance coverage to participants, ensuring they fully understand their rights and protections. Regular reviews and updates of insurance policies in accordance with regulatory changes are essential for maintaining compliance and ensuring the welfare of participants. By prioritizing these measures, sponsors can foster a safer clinical trial environment.
Navigating the landscape of clinical trial agreements in Bulgaria requires a deep understanding of the regulatory framework and ethical considerations that are essential for successful research. By following key practices, stakeholders can not only ensure compliance with local laws but also cultivate a trustworthy environment for both participants and researchers.
Crucial practices include:
Each of these components is vital for maintaining the integrity of clinical research and safeguarding the rights of individuals involved in trials.
The importance of these practices goes beyond mere compliance; they are instrumental in advancing medical knowledge and innovation. By prioritizing ethical standards and regulatory adherence, researchers can bolster the credibility of their studies and foster a culture of safety and transparency in clinical trials. Engaging with these best practices not only benefits the research community but also protects the welfare of participants, ensuring that Bulgaria remains a viable and ethical location for clinical trials.
What is the significance of the EU Clinical Studies Regulation in Bulgaria?
The EU Clinical Studies Regulation (EU No 536/2014) is crucial for conducting clinical studies in Bulgaria as it outlines the necessary requirements for testing approval, including submission methods and ethical considerations, ensuring compliance with EU and national standards.
How must sponsors submit their applications for clinical trials in Bulgaria?
Sponsors must submit their applications through the Clinical Trials Information System (CTIS), a platform designed to streamline the approval process and enhance efficiency.
Why is it important to stay informed about changes to clinical trial legislation in Bulgaria?
Staying informed about changes to legislation is vital because updates can significantly affect court timelines and requirements for clinical trials.
What role does the Bulgarian Drug Agency (BDA) play in clinical trials?
The BDA oversees medical trials to ensure they meet high safety and quality standards, creating a trustworthy environment for innovative research.
What is informed consent in the context of clinical research?
Informed consent is a fundamental ethical requirement that ensures potential participants are fully informed about the study's purpose, procedures, risks, and benefits before agreeing to participate.
What language must consent forms be drafted in for clinical trials in Bulgaria?
Consent forms must be drafted in Bulgarian and adhere to local regulatory standards.
How can researchers enhance participants' understanding of the consent process?
Researchers can enhance understanding by using plain language, avoiding technical jargon, and providing ample opportunities for participants to ask questions.
Why is meticulous documentation of the consent procedure important?
Meticulous documentation serves as legal protection and provides proof of adherence to ethical standards, honoring individuals' rights and enhancing the integrity of the research process.