


The article outlines five proven strategies for successful clinical trial patient enrollment. It emphasizes the importance of:
Evidence supports these strategies, indicating that addressing common recruitment challenges can significantly improve enrollment rates and ensure diverse representation. Ultimately, these efforts enhance the reliability of clinical trial data and advance medical research.
Clinical trial patient enrollment is a pivotal element in the success of medical research, directly impacting the quality of data and the timely introduction of new therapies. Despite its significance, many trials face recruitment challenges that often result in delays and increased costs.
What strategies can be employed to overcome these barriers and ensure robust participant engagement? This article explores five proven methods that not only enhance enrollment success but also foster a more inclusive representation in clinical studies. Ultimately, these strategies advance medical innovation and improve patient care.
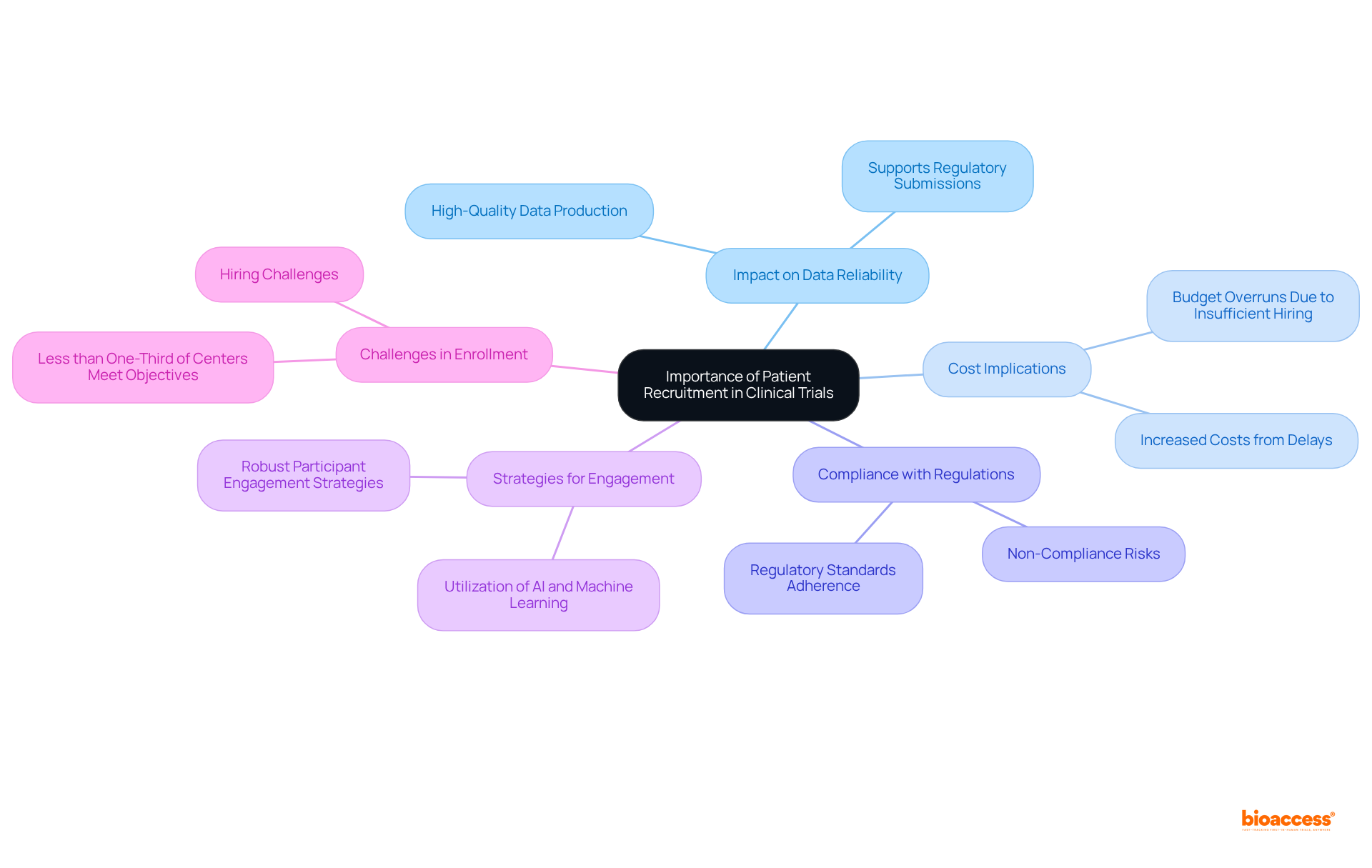
Clinical trial patient enrollment is the cornerstone of clinical studies' success, significantly influencing the reliability of data and the timelines for introducing new treatments to the market. Insufficient hiring can lead to substantial delays, increased costs, and potential non-compliance with regulatory standards. Research indicates that trials employing robust participant engagement strategies are more likely to achieve their enrollment goals and complete on schedule, ultimately improving patient care and advancing medical knowledge.
Moreover, effective clinical trial patient enrollment is crucial for preserving the integrity of medical research. Trials with well-defined strategies for clinical trial patient enrollment produce high-quality data that supports regulatory submissions and informs clinical practice. As highlighted by industry experts, hiring challenges remain a significant barrier, with less than one-third of research centers meeting their hiring objectives. This underscores the necessity for all stakeholders—sponsors, contract research organizations (CROs), and regulatory bodies—to prioritize participant engagement strategies, thereby enhancing success rates and fostering the advancement of medical innovations.
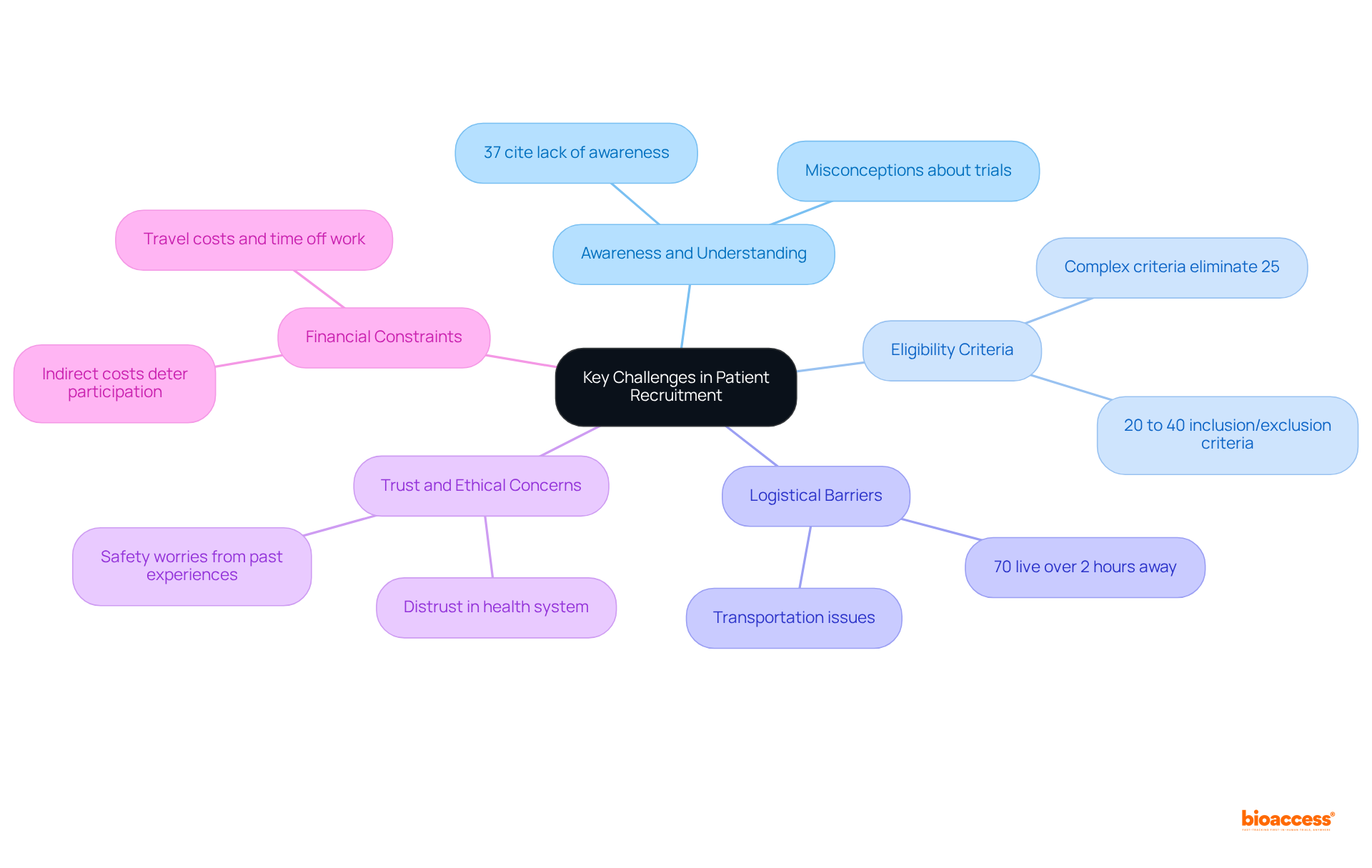
Effective patient recruitment in clinical trials is often hindered by several key challenges:
Awareness and Understanding: A significant percentage of potential participants remain uninformed about clinical studies. Research indicates that nearly 37% of investigators cite a lack of awareness as a major obstacle. This gap in knowledge can foster misconceptions, leading to reluctance in participation.
Eligibility Criteria: The complexity of eligibility requirements can severely restrict the pool of potential participants. Clinical studies typically include 20 to 40 inclusion and exclusion criteria, which can eliminate up to 25% of interested individuals, complicating recruitment efforts.
Logistical Barriers: Geographic distance from research locations presents a significant challenge. Around 70% of eligible individuals reside more than two hours away from a study center. Transportation issues and the burden of frequent visits can deter participation, particularly for those with limited mobility or financial resources.
Trust and Ethical Concerns: Patients frequently possess worries about the safety and ethical ramifications of participating in studies, especially if they have previously faced adverse experiences. This distrust can significantly impact their willingness to enroll in studies.
Financial Constraints: The financial burden associated with participation, including travel costs and time off work, can be prohibitive. Numerous individuals encounter indirect expenses that hinder involvement, further complicating the process of attracting participants.
By identifying and tackling these obstacles, study sponsors and contract research entities can create focused engagement approaches that improve enrollment success and guarantee diverse participant representation.
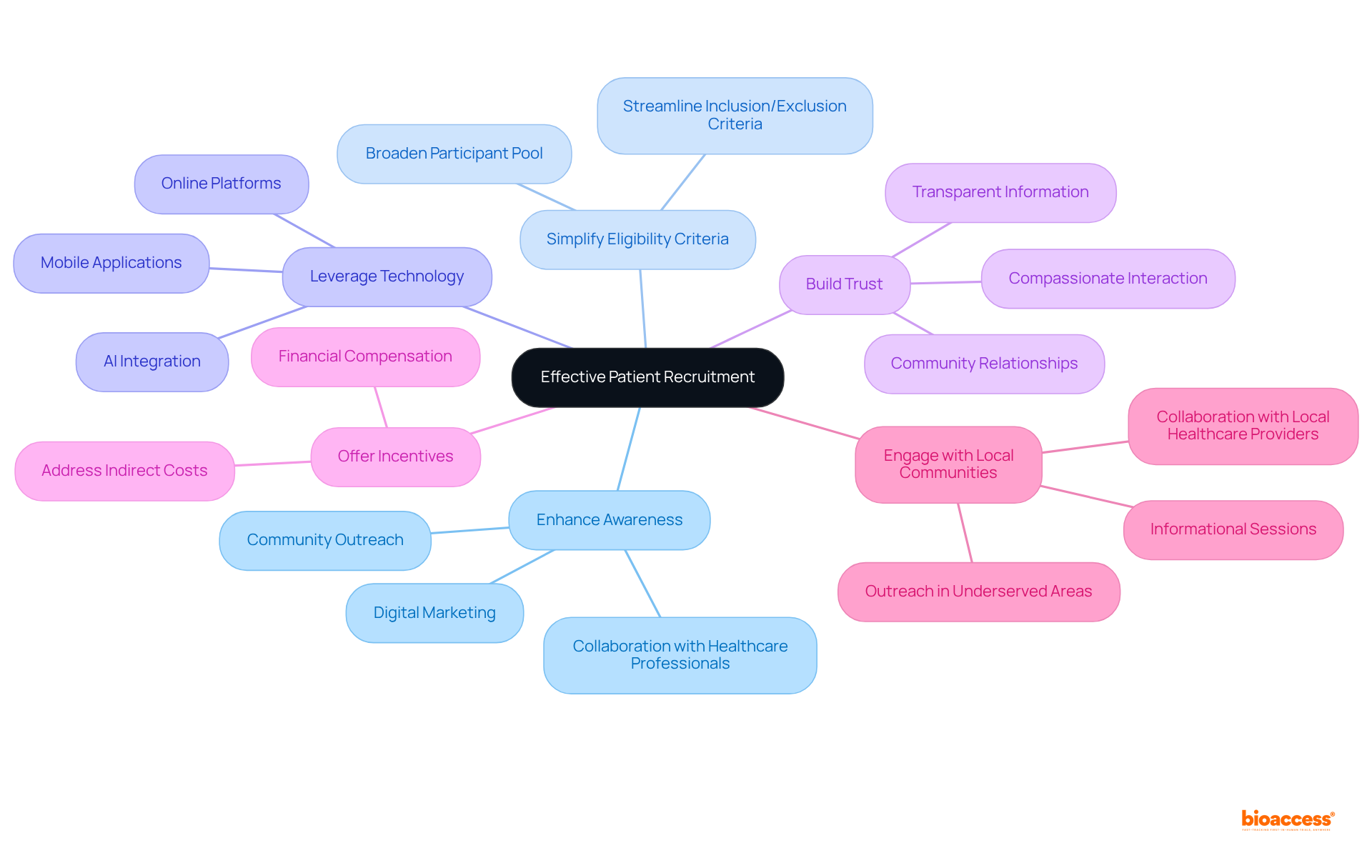
To enhance clinical trial patient enrollment, implementing proven strategies is essential.
Enhance Awareness: Utilize digital marketing, social media, and community outreach to increase knowledge about research studies. Collaborating with healthcare professionals to educate individuals about current studies can significantly elevate involvement rates. Given that 86% of international studies fail to meet participant goals within the appropriate timeframe, raising awareness is crucial.
Simplify Eligibility Criteria: Streamlining inclusion and exclusion criteria can broaden the pool of eligible participants, attracting a more diverse participant base. This approach addresses the issue that many patients are unaware of their eligibility to participate in clinical trial patient enrollment, which often obstructs the efforts to enroll participants.
Leverage Technology: Online platforms and mobile applications can streamline the recruitment process. These tools enable potential participants to learn about trials, pre-screen for eligibility, and even enroll online. The integration of AI and digital solutions has shown promise in enhancing recruitment efficiency, allowing for quicker identification of suitable individuals.
Build Trust: Establishing relationships with community members and advocacy groups is vital for fostering trust. Providing clear, transparent information about the process and actively addressing individuals' concerns can alleviate fears and enhance engagement. Compassionate interaction is essential, as it significantly improves experiences and outcomes in research studies.
Offer Incentives: Providing financial compensation for travel and time, along with other incentives, can make participation more appealing. Addressing indirect costs associated with participation, such as travel and lost income, can help mitigate barriers for disadvantaged populations.
Engage with Local Communities: Conducting outreach in local communities, particularly in underserved areas, ensures diverse representation in studies. This can involve hosting informational sessions and collaborating with local healthcare providers. Involving previously underrepresented patients enhances the quality of data gathered and ensures that research studies reflect the real-world population.
By adopting these approaches, study sponsors can significantly bolster their clinical trial patient enrollment efforts, ensuring that studies are sufficiently robust and representative of the population.
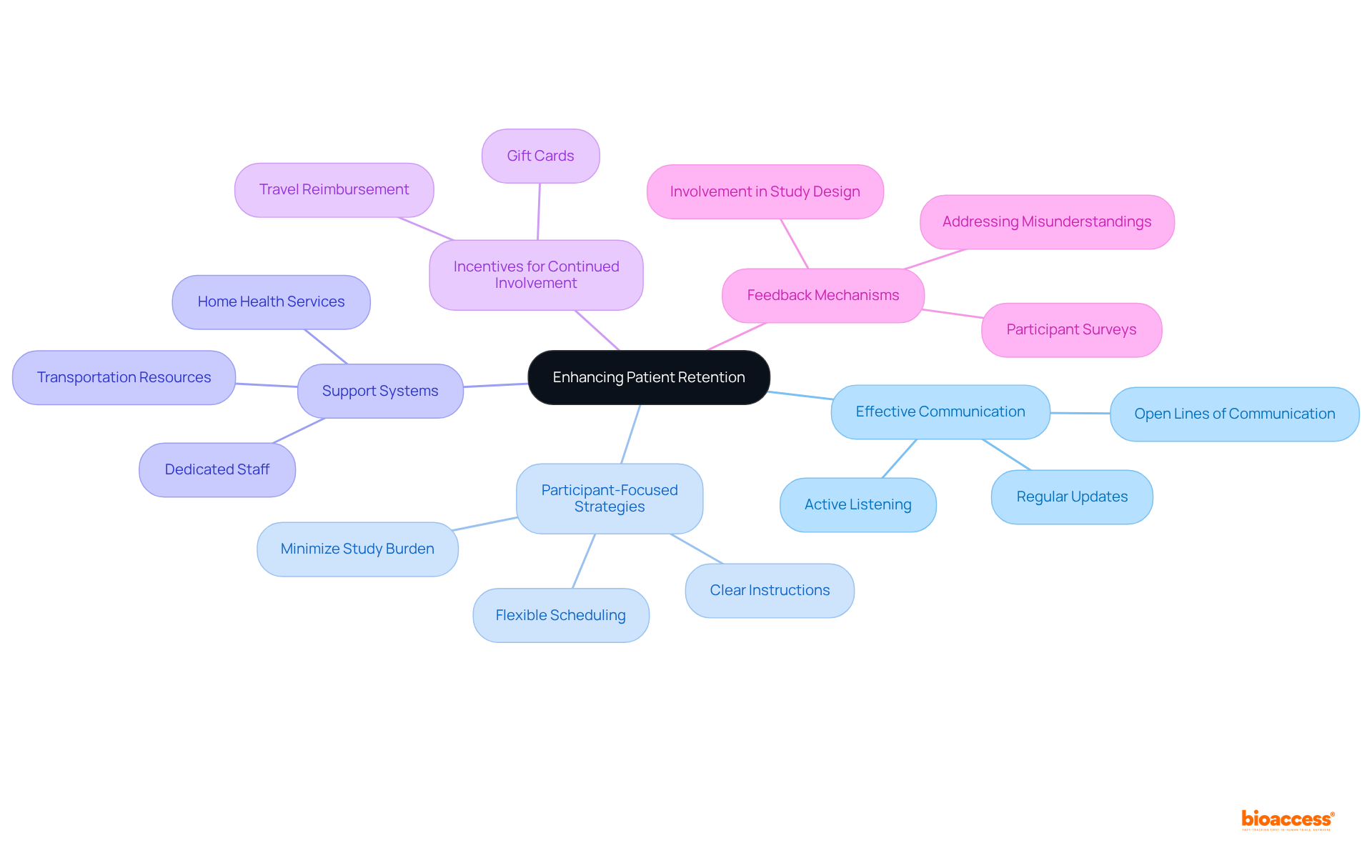
Patient retention is as crucial as recruitment in ensuring the success of clinical studies. High dropout rates can compromise the integrity of the study and lead to inconclusive results. To enhance patient retention, consider the following strategies:
Effective Communication: Maintain open lines of communication with participants throughout the study. Regular updates and check-ins can help keep participants engaged and informed about their contributions. Research indicates that consistent communication can significantly enhance participant commitment, thereby reducing dropout rates. As noted, "Consistent communication and involvement with participants can enhance their dedication to the study, decreasing drop-out rates."
Participant-Focused Strategies: Create studies with the individual's experience in mind. This includes minimizing the burden of study visits, offering flexible scheduling, and providing clear instructions about what to expect. Patient-centered studies have demonstrated the ability to enlist the required number of patients in nearly half the time compared to conventional approaches.
Support Systems: Establish support systems for participants, such as dedicated staff to address questions and concerns. Providing resources for transportation or accommodations can alleviate logistical burdens. Implementing home health services can further reduce travel-related stress, thus enhancing retention.
Incentives for Continued Involvement: Consider providing incentives for participants who complete the study, such as gift cards or reimbursement for travel costs. This can motivate participants to remain engaged, as financial support for travel has been shown to improve attendance.
Feedback Mechanisms: Implement feedback mechanisms to understand participants' experiences and address any issues that may arise during the study. Involving individuals in the study design process can yield valuable insights and enhance retention rates, as participants feel their opinions are valued. Additionally, recognizing frequent misunderstandings regarding medical studies can help tailor communication approaches effectively.
By focusing on clinical trial patient enrollment, study sponsors can ensure that research is completed promptly and delivers reliable, high-quality information. With dropout rates averaging around 7 out of 31 enrolled participants, and some trial designers accounting for dropout rates as high as 30% when budgeting and planning for a trial, prioritizing strategies for clinical trial patient enrollment is essential for the success of clinical trials.
The success of clinical trials hinges significantly on effective patient enrollment strategies, which are crucial for ensuring reliable data and timely market introduction of new treatments. Recognizing the importance of patient recruitment allows stakeholders to address the challenges faced and implement robust strategies that enhance enrollment while contributing to the integrity of medical research.
Key challenges—such as lack of awareness, complex eligibility criteria, logistical barriers, trust issues, and financial constraints—are major obstacles to successful patient recruitment. However, by adopting proven strategies, including:
research sponsors can significantly improve their enrollment efforts. Additionally, focusing on patient retention through effective communication, participant-centered strategies, and support systems further strengthens the overall success of clinical trials.
Ultimately, the implications of successful patient recruitment extend beyond individual studies; they foster advancements in medical knowledge and patient care. By prioritizing these strategies, stakeholders can enhance enrollment and retention rates while ensuring that clinical trials reflect the diverse populations they aim to serve. Embracing these best practices is essential for overcoming barriers and achieving research goals that ultimately benefit society as a whole.
Why is patient recruitment important in clinical trials?
Patient recruitment is crucial because it influences the reliability of data and the timelines for introducing new treatments to the market. Insufficient recruitment can lead to delays, increased costs, and potential non-compliance with regulatory standards.
How does participant engagement impact clinical trial success?
Robust participant engagement strategies increase the likelihood of achieving enrollment goals and completing trials on schedule, which ultimately improves patient care and advances medical knowledge.
What are the consequences of inadequate patient recruitment in clinical trials?
Inadequate patient recruitment can result in substantial delays, increased costs, and potential issues with regulatory compliance.
What role do participant engagement strategies play in clinical trials?
Participant engagement strategies are essential for preserving the integrity of medical research and producing high-quality data that supports regulatory submissions and informs clinical practice.
What challenges do research centers face in patient recruitment?
Many research centers face hiring challenges, with less than one-third meeting their recruitment objectives, highlighting the need for improved participant engagement strategies.
Who should prioritize participant engagement strategies in clinical trials?
All stakeholders, including sponsors, contract research organizations (CROs), and regulatory bodies, should prioritize participant engagement strategies to enhance success rates and foster the advancement of medical innovations.