


Establishing a Data Monitoring Board (DMB) is essential for ensuring the integrity and safety of clinical studies, especially in the dynamic realm of Macedonian medical research. This specialized group not only protects participant welfare but also bolsters the credibility of research findings, aligning local practices with international standards. Yet, the challenge lies in navigating complex regulatory requirements and assembling a board with the right expertise to fulfill its crucial role.
How can researchers effectively set up and sustain a DMB that meets these demands while fostering innovation and trust in the research process? This question is pivotal as it highlights the need for a strategic approach to DMB establishment, ensuring that it not only complies with regulations but also enhances the overall research landscape.
A Data Monitoring Board (DMB) plays a pivotal role in the supervision of clinical studies, focusing on participant safety, treatment effectiveness, and study integrity. This specialized group of experts is tasked with overseeing collected data, ensuring that any potential risks to participants are promptly addressed. Operating independently from study sponsors and researchers, the DMB provides essential impartial oversight, fostering public confidence in medical research. Decisions regarding the continuation or modification of a study are made solely based on data and ethical considerations.
In Macedonia, where the medical research landscape is rapidly evolving, the data monitoring board setup in Macedonian studies is essential for compliance with local regulations and alignment with international standards. The presence of a DMB not only enhances participant safety but also bolsters the credibility of the research process, ultimately contributing to the advancement of medical knowledge and innovation. To ensure comprehensive oversight, the DMB should include a statistician who can offer independent statistical expertise, develop operational procedures such as voting rules and attendance guidelines, and produce both Open Session and Closed Session Reports to maintain transparency.
Moreover, staggered terms for standing DMBs are crucial for ensuring continuity and effective governance. By integrating extensive research study management services - including feasibility assessments, site selection, compliance evaluations, study setup, and project management - bioaccess guarantees that the DMB operates within a robust framework that supports the overall success of research initiatives in the region.

The data monitoring board setup in Macedonian studies is crucial for ensuring compliance with regulatory frameworks that align with European Union standards. The Law on Personal Data Protection, along with guidelines from the Agency for Medicines and Medical Devices of Macedonia, plays a pivotal role in this context. These regulations mandate that DMBs include members with relevant expertise, such as clinicians and biostatisticians, to guarantee comprehensive oversight of medical studies.
Moreover, the DMB must operate under a charter that clearly outlines its responsibilities, meeting frequency, and reporting procedures. Starting July 1, 2025, compliance with personal data security standards will be mandatory, compelling organizations to evaluate their readiness for these changes. Understanding these regulations is vital for ensuring that the data monitoring board setup in Macedonian studies operates within the legal framework, thereby safeguarding the integrity of research studies and enhancing participant safety.
It's equally important for DMB members to maintain independence from research investigators, both intellectually and financially, to uphold objectivity in their oversight. Bioaccess stands ready to assist organizations in navigating these regulatory requirements effectively, offering comprehensive trial management services that include:
By collaborating with Bioaccess, organizations can address key challenges in the Medtech landscape and ensure their DMB functions optimally.
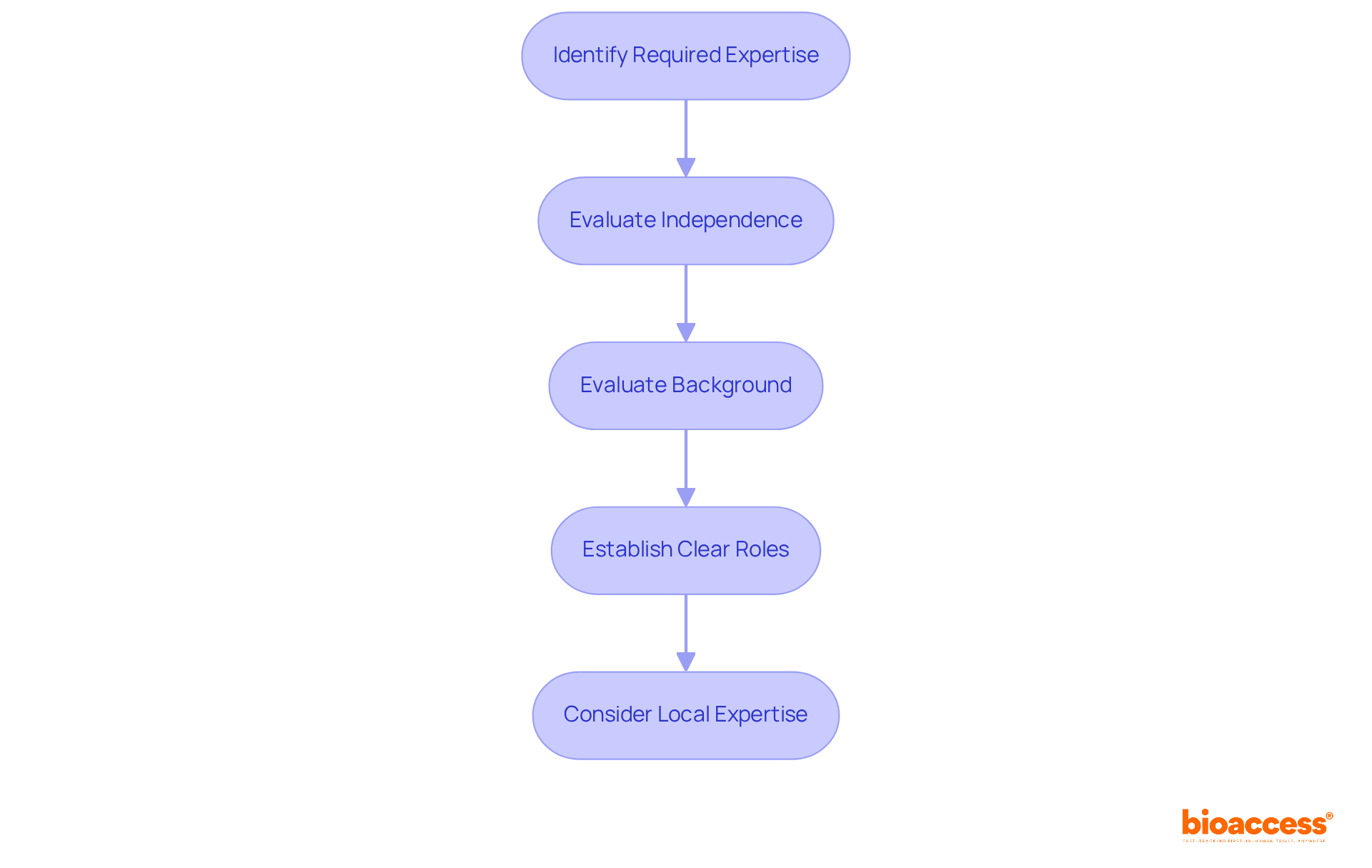
When assembling your Data Monitoring Board (DMB), it's crucial to follow these steps:
Identify Required Expertise: Ensure your DMB comprises members with diverse backgrounds, including clinical researchers, biostatisticians, and ethicists. This diversity enhances the board's capacity to evaluate information from various perspectives, fostering comprehensive assessments.
Evaluate Independence: Choose individuals who have no financial or personal connections to the sponsor or researchers. This independence is vital for maintaining objectivity and integrity in the board's evaluations.
Evaluate Background: Favor individuals with considerable experience in clinical studies and oversight. Their knowledge is essential for navigating complex information and making informed choices that uphold the study's scientific rigor.
Establish Clear Roles: Clearly define the roles and responsibilities of each board member. This structured approach facilitates effective communication and decision-making, ensuring that all members understand their contributions to the DMB's objectives.
Consider Local Expertise: In Macedonia, including members familiar with the regional healthcare landscape and regulatory environment is essential. Their insights can significantly enhance the board's effectiveness in overseeing trials conducted in the area.
To establish effective operational protocols for your Data Monitoring Board (DMB), follow these essential steps:
Develop a Charter: Create a comprehensive charter that clearly defines the DMB's purpose, responsibilities, and operational procedures. This document should outline guidelines for information review, meeting frequency, and reporting requirements, ensuring clarity in roles and expectations.
Set Meeting Schedules: Establish a regular meeting timetable for the DMB to review information and make informed decisions. Frequent meetings are vital for timely oversight and prompt intervention when necessary, fostering a proactive approach to participant safety. The structured meeting format should include Open, Closed, and Closed Executive Sessions to facilitate comprehensive discussions.
Establish Review Procedures: Implement clear protocols for evaluation, including specific criteria for assessing safety and efficacy. These protocols should also address how to manage adverse events and unforeseen issues, ensuring a robust framework for integrity in the study.
Document Everything: Maintain thorough records of all meetings, decisions, and evaluations. This record-keeping is essential for regulatory compliance and enhances transparency throughout the trial process, reinforcing trust among stakeholders. The DMB is responsible for approving written minutes that summarize discussions and findings, which is crucial for maintaining confidentiality and managing conflicts of interest.
By following these steps, you can ensure that your data monitoring board setup in Macedonian studies operates effectively and upholds the highest standards of safety and ethical oversight. With the demand for information specialists increasing by 36%, having skilled individuals in your DMB is more crucial than ever.

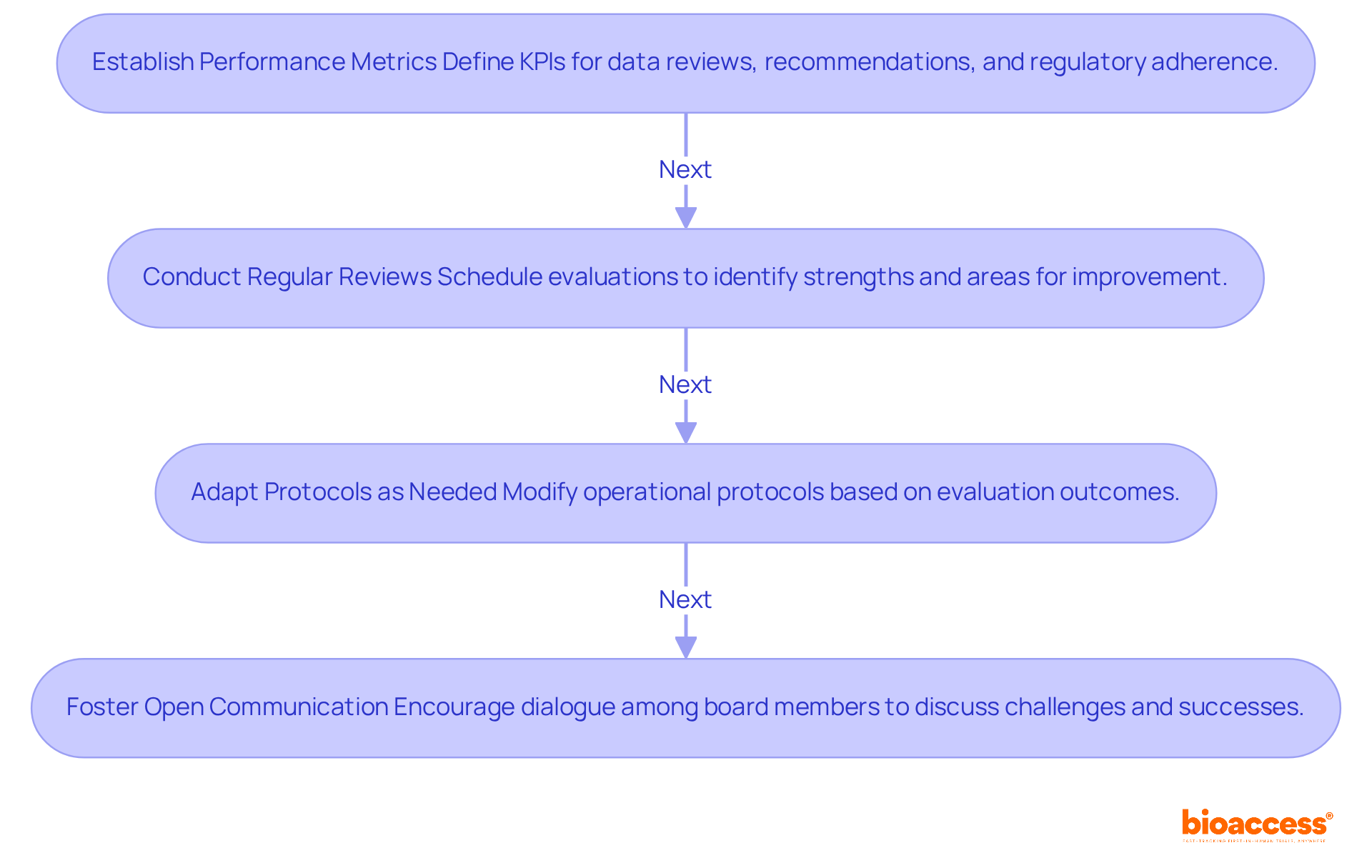
To effectively evaluate and enhance your Data Monitoring Board's (DMB) performance, it's crucial to follow these essential steps:
Establish Performance Metrics: Begin by defining key performance indicators (KPIs) such as the timeliness of data reviews, the accuracy of recommendations, and adherence to regulatory standards. These metrics are vital for assessing the board's impact on study integrity and patient safety. Notably, nearly 30% of participants withdraw after enrollment, underscoring the need for effective oversight.
Conduct Regular Reviews: Implement a schedule for periodic evaluations of the DMB's performance, incorporating feedback from board members and stakeholders. This practice not only identifies strengths but also highlights areas needing improvement, ensuring the board remains aligned with trial objectives. Frequent evaluations can lead to innovative solutions and enhanced oversight, which are essential for safeguarding patient safety and maintaining information integrity.
Adapt Protocols as Needed: Be ready to modify operational protocols based on evaluation outcomes. This may involve revising meeting frequencies, streamlining data review processes, or redefining member roles to boost efficiency and responsiveness. It's also important to consider the requirement for ethics committee approvals before commencing studies in these adaptations to ensure compliance with regulatory standards.
Foster Open Communication: Cultivate a culture of open dialogue among board members to discuss challenges and successes. This collaborative environment not only encourages innovative solutions but also strengthens oversight, ultimately leading to improved trial outcomes. Experts emphasize that effective decision-making hinges on evaluating available information and integrating insights from diverse perspectives. Regular monitoring of these factors can significantly enhance the effectiveness of the data monitoring board setup in Macedonian studies within clinical research.
Establishing a Data Monitoring Board (DMB) is crucial for ensuring the integrity and safety of clinical studies, especially in the dynamic landscape of Macedonian medical research. This independent oversight not only protects participant welfare but also bolsters the credibility of research initiatives, aligning them with local regulations and international standards.
The article outlines essential steps for setting up a DMB:
Each component is vital for creating a robust framework that supports ethical research practices and fosters public trust in clinical trials.
Ultimately, the establishment of a DMB transcends mere regulatory compliance; it represents a significant commitment to ethical standards and participant safety. Organizations in Macedonia are urged to prioritize the formation of effective DMBs, leveraging expert insights and operational best practices to navigate challenges in the Medtech landscape. By doing so, they can contribute to the advancement of medical knowledge while ensuring the highest standards of data integrity and participant protection are upheld.
What is the role of a Data Monitoring Board (DMB)?
A Data Monitoring Board (DMB) supervises clinical studies, focusing on participant safety, treatment effectiveness, and study integrity. It oversees collected data and addresses potential risks to participants while operating independently from study sponsors and researchers.
Why is a DMB important for clinical studies?
A DMB provides impartial oversight, fostering public confidence in medical research. It makes decisions about the continuation or modification of studies based on data and ethical considerations, enhancing participant safety and the credibility of the research process.
What expertise should members of a DMB possess?
DMB members should include experts such as clinicians and biostatisticians to ensure comprehensive oversight of medical studies. Additionally, having a statistician is crucial for independent statistical expertise.
What regulatory requirements must DMBs in Macedonia comply with?
DMBs in Macedonia must comply with the Law on Personal Data Protection and guidelines from the Agency for Medicines and Medical Devices of Macedonia. This includes having members with relevant expertise and operating under a charter that outlines responsibilities and reporting procedures.
When will compliance with personal data security standards become mandatory in Macedonia?
Compliance with personal data security standards will be mandatory starting July 1, 2025.
How can organizations ensure their DMB operates effectively?
Organizations can ensure effective DMB operation by integrating extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, and project management.
What is the importance of maintaining independence for DMB members?
DMB members must maintain independence from research investigators, both intellectually and financially, to uphold objectivity in their oversight of clinical studies.
How can Bioaccess assist organizations with DMBs?
Bioaccess can help organizations navigate regulatory requirements and provide comprehensive trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.