


This article delineates five essential steps for the effective design of a clinical trial:
Each of these steps is pivotal in ensuring that the trial is structured to meet both regulatory standards and scientific objectives. This structured approach ultimately facilitates the successful evaluation of medical interventions, underscoring the importance of meticulous planning in clinical research.
Designing a clinical trial is a complex yet vital process that serves as the cornerstone of advancing medical science and ensuring patient safety. By meticulously navigating through the phases of clinical trials, researchers can uncover the safety and efficacy of new treatments, ultimately shaping the future of healthcare. The challenge, however, lies in balancing rigorous scientific standards with ethical considerations and regulatory compliance.
How can researchers effectively structure their trials to meet these demands while also achieving meaningful results?
Clinical trials represent systematic investigations designed to evaluate the safety and efficacy of medical interventions, progressing through four distinct phases:
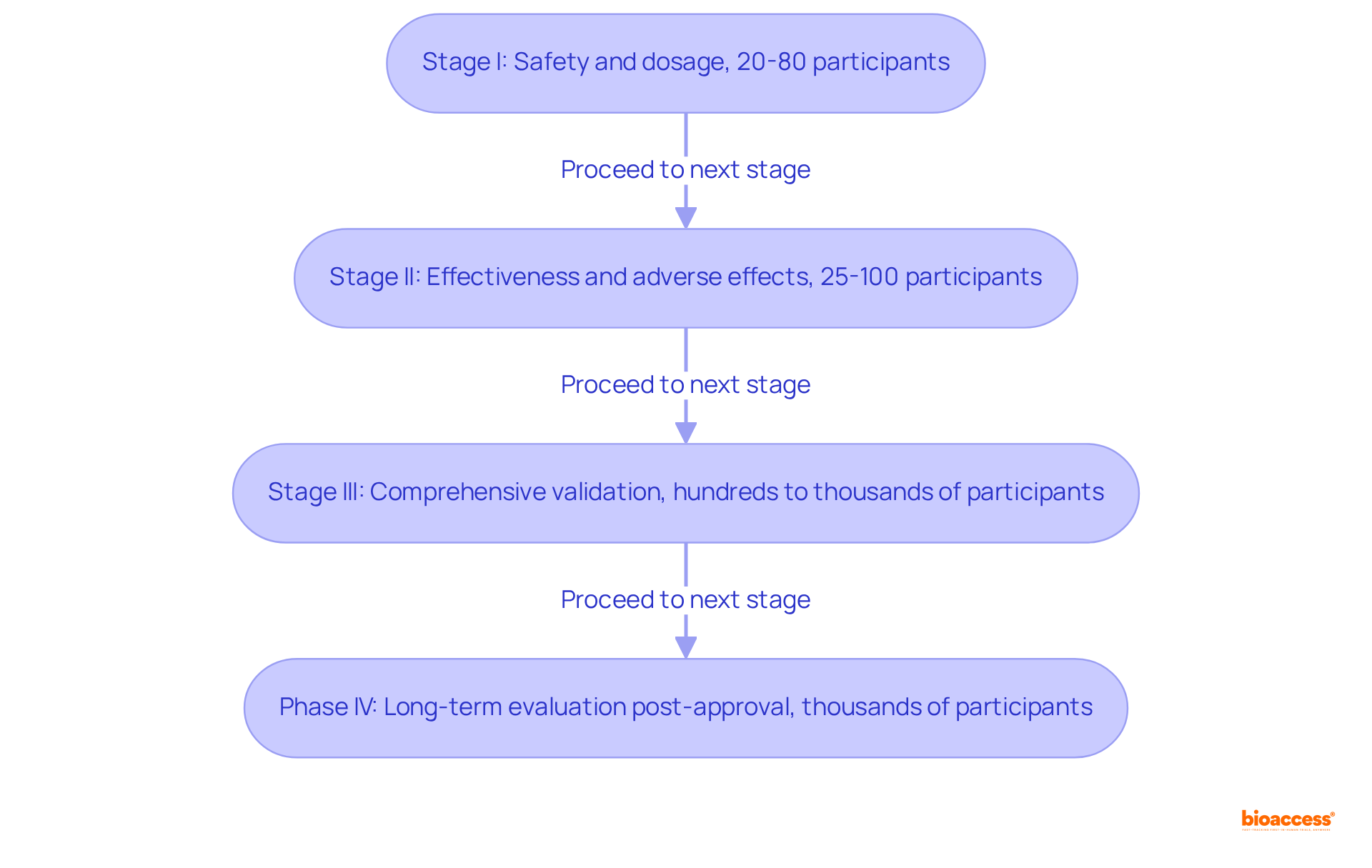
Stage I: This initial stage emphasizes safety and dosage, generally involving 20 to 80 healthy participants. It aims to establish the safety profile of a therapy and determine the highest safe dose, with close monitoring for side effects. Notably, about 70% of medications that enter Stage I trials successfully advance to Stage II.
Stage II: During this stage, the effectiveness and adverse effects are assessed with a larger group of 25 to 100 individuals who have the specific condition. This phase is crucial for evaluating whether the intervention demonstrates adequate benefit and tolerable side effects, as approximately 33% of therapies that commence Stage II progress to Stage III. Importantly, Stage II studies do not utilize placebos (inactive therapies).
Stage III: These studies are the most comprehensive, involving several hundred to several thousand participants. They validate the method's effectiveness, observe side effects, and contrast it with standard approaches. Successful Stage III studies can result in regulatory endorsement, providing essential information on the method's relative efficacy and safety.
Phase IV: Conducted after a therapy has received market approval, Phase IV studies gather additional information on long-term risks, benefits, and optimal use in the general population. These studies frequently include thousands of participants and yield valuable insights for ongoing safety evaluations.
Understanding these phases is crucial for structuring a clinical study that can help design a clinical trial which meets both regulatory standards and scientific objectives, ultimately aiding the successful progression of innovative medical therapies. Furthermore, bioaccess® facilitates ethical approvals in 4-6 weeks and achieves enrollment 50% quicker than conventional markets, thereby enhancing the effectiveness of clinical studies.
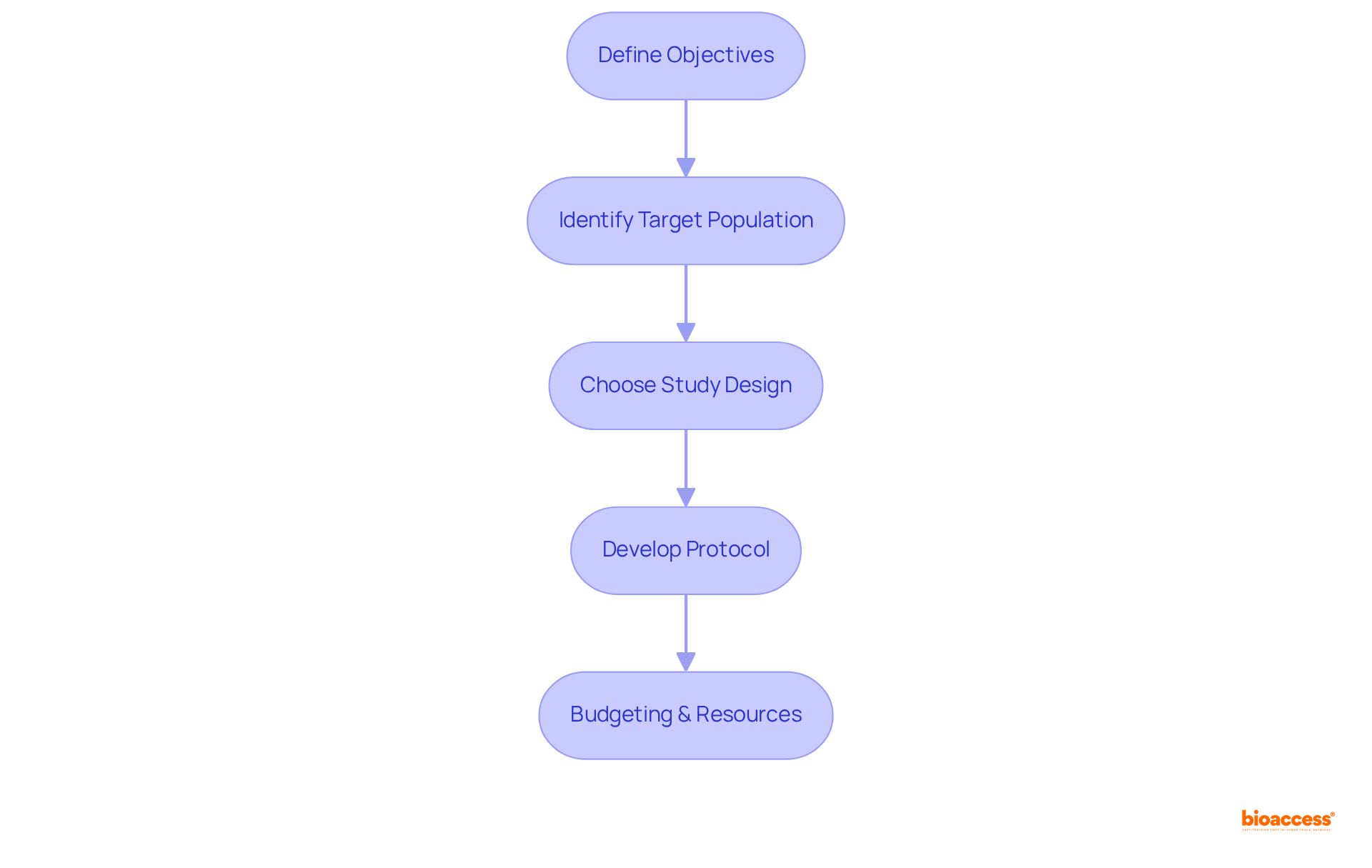
The key steps to design a clinical trial are essential for ensuring a successful outcome. To begin, it is crucial to define the objectives; clearly articulating both the primary and secondary goals of the study sets a solid foundation. Next, identifying the target population involves determining the specific characteristics of the participants who will be included in the study, ensuring that the results are applicable and relevant. Choosing the appropriate study design is also vital; this decision could range from a randomized controlled experiment to an observational study, each serving different research purposes. Additionally, developing a comprehensive protocol is necessary; this document should outline the methodology in detail, covering care plans, assessments, and timelines. Finally, budgeting and resources must be carefully estimated, identifying necessary resources such as personnel and facilities. Following these steps provides a structured method to design a clinical trial, emphasizing the importance of a meticulous approach in clinical research.
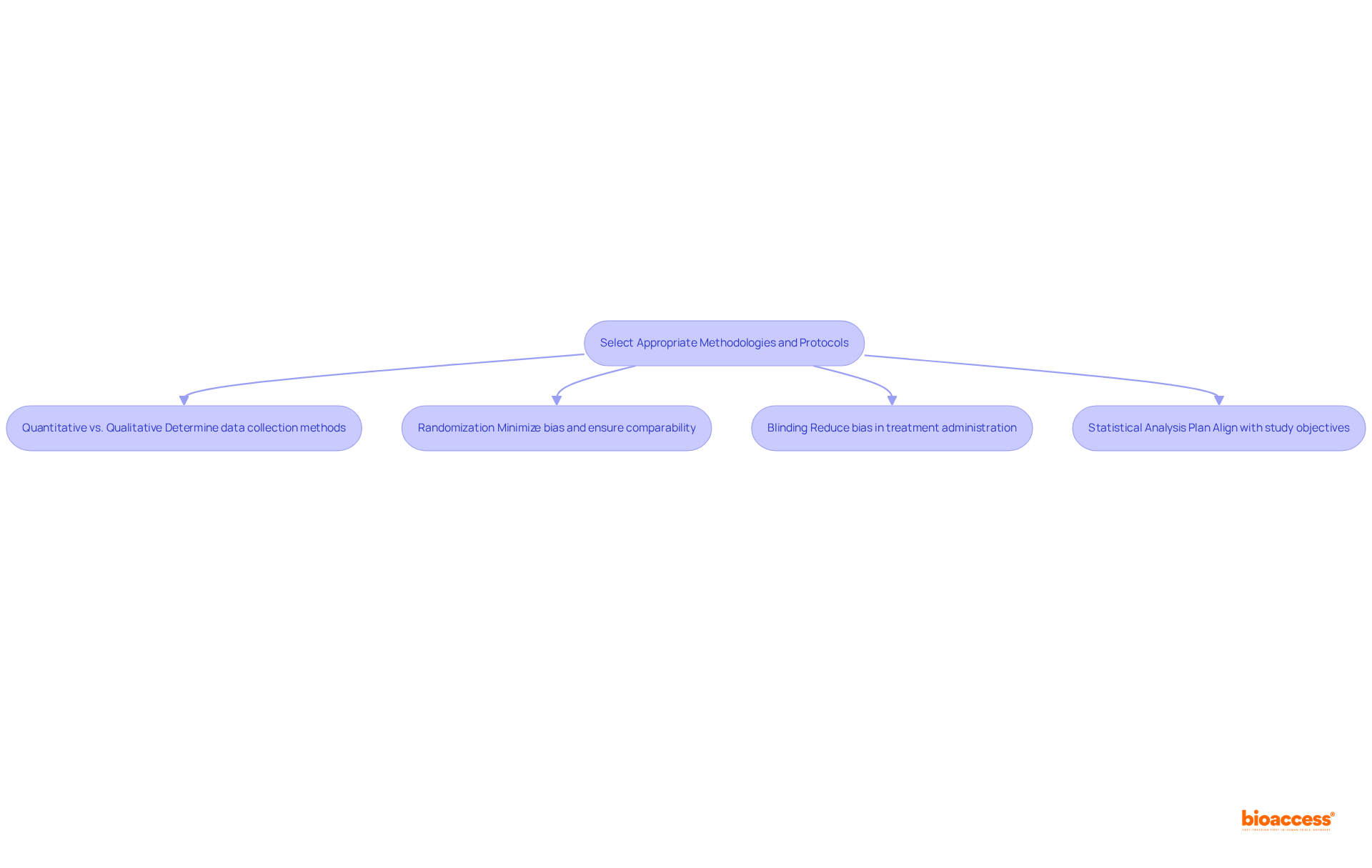
Selecting appropriate methodologies is crucial to design a clinical trial in clinical research. It involves several key considerations:
Selecting the appropriate methodologies is essential to design a clinical trial that not only improves the credibility of the study findings but also upholds the integrity and reliability of the research outcomes.
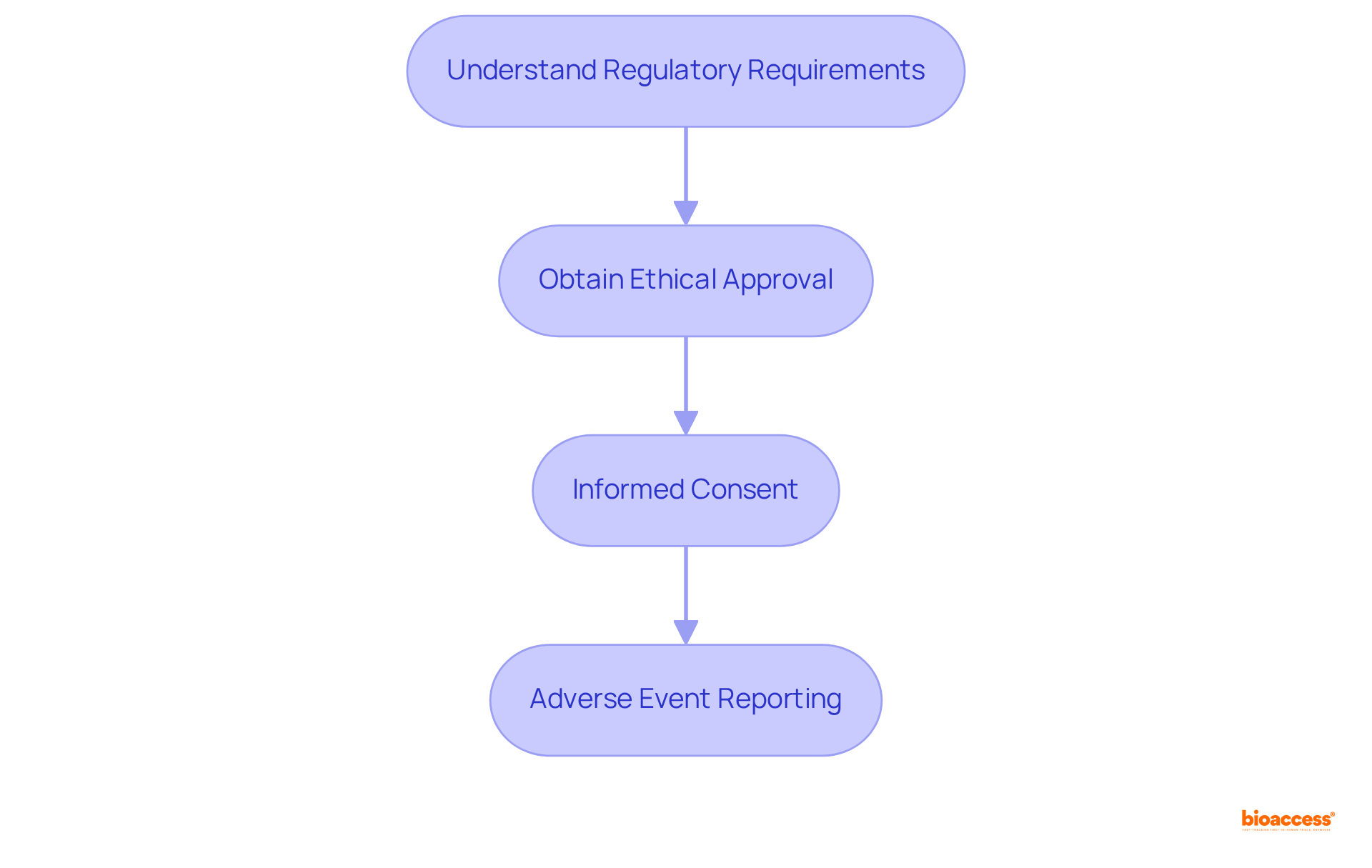
To ensure compliance with regulatory and ethical standards in clinical trials, it is imperative to follow these essential steps:
Adhering to these standards is critical for maintaining ethical conduct and achieving regulatory approval, which is essential when we design a clinical trial to ensure the successful advancement of clinical research. Moreover, addressing medical mistrust, particularly within BIPOC communities, is crucial for enhancing representation and confidence in clinical studies.
To implement effective strategies in clinical research, it is essential to design a clinical trial with a clear framework that addresses key challenges.
These strategies not only enhance participant engagement but also ensure the reliability of collected data, reinforcing the importance of a systematic approach to design a clinical trial in clinical research.
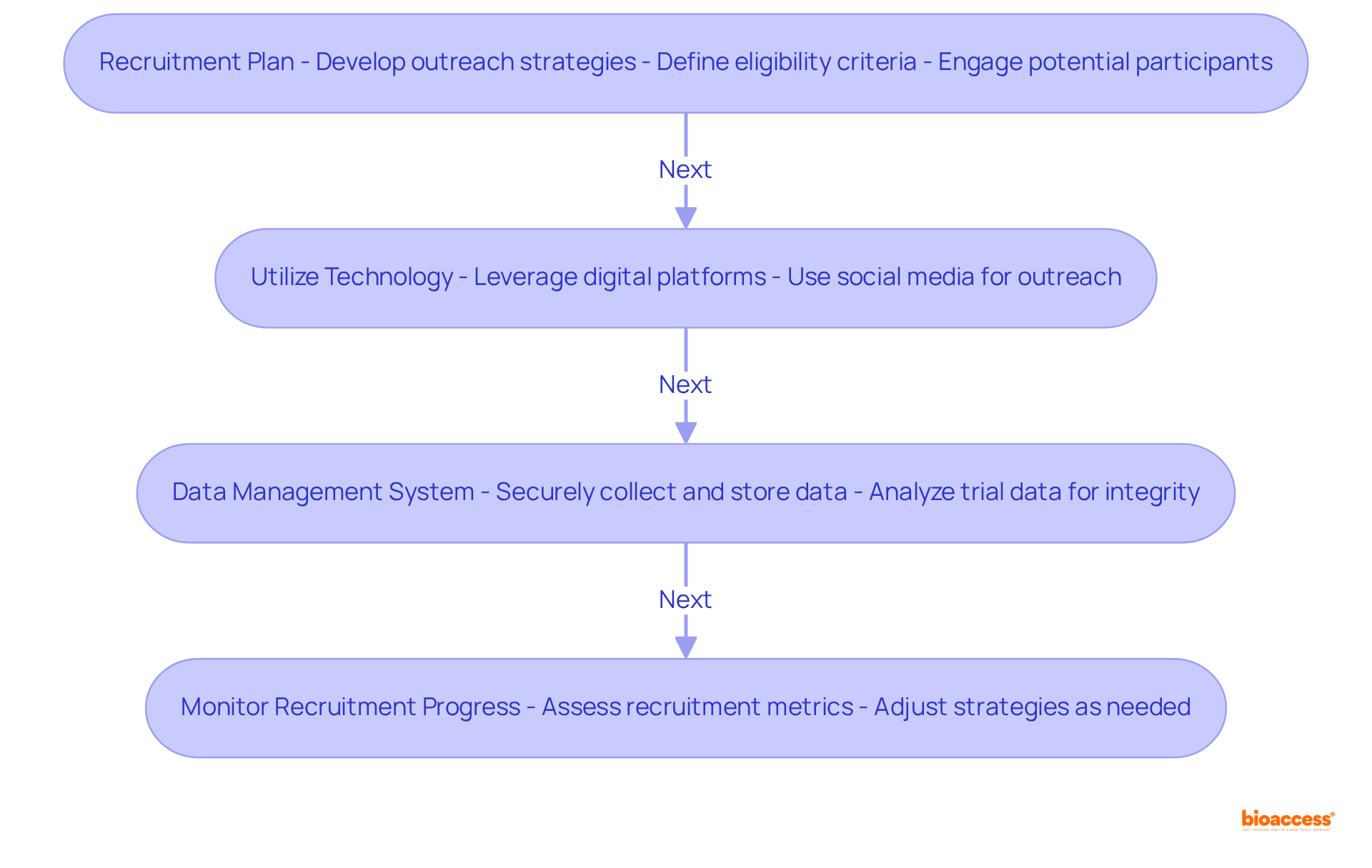
Designing a clinical trial effectively is a multifaceted process that demands careful consideration of various elements to ensure successful outcomes. This article outlines essential steps, emphasizing the importance of:
Each component plays a critical role in the overall integrity and effectiveness of the trial.
Key insights reveal the necessity of a structured approach to trial design, encompassing everything from initial safety assessments to long-term evaluations of therapeutic effectiveness. By meticulously defining objectives, identifying target populations, and ensuring compliance with ethical standards, researchers can enhance the credibility and reliability of their findings. Furthermore, leveraging technology and robust data management systems significantly improves participant engagement and data integrity throughout the trial.
Ultimately, the significance of effective clinical trial design cannot be overstated. It serves as the backbone for advancing medical research and ensuring that new therapies are both safe and effective. By following the outlined steps and embracing best practices, researchers contribute to the evolution of clinical science, ultimately leading to improved patient outcomes and greater trust in the medical research process. Engaging in this meticulous design process not only fosters innovation but also ensures that the voices of diverse populations are heard and represented in clinical research.
What are clinical trials?
Clinical trials are systematic investigations designed to evaluate the safety and efficacy of medical interventions, progressing through four distinct phases.
What happens in Stage I of a clinical trial?
Stage I focuses on safety and dosage, involving 20 to 80 healthy participants. It aims to establish the safety profile of a therapy and determine the highest safe dose, with close monitoring for side effects. About 70% of medications that enter Stage I trials successfully advance to Stage II.
What is the purpose of Stage II in clinical trials?
Stage II assesses the effectiveness and adverse effects of the intervention with a larger group of 25 to 100 individuals who have the specific condition. This phase evaluates whether the intervention demonstrates adequate benefit and tolerable side effects, with approximately 33% of therapies progressing to Stage III. Stage II studies do not use placebos.
What occurs during Stage III of a clinical trial?
Stage III studies are comprehensive and involve several hundred to several thousand participants. They validate the method's effectiveness, observe side effects, and compare it with standard approaches. Successful Stage III studies can lead to regulatory endorsement.
What is the focus of Phase IV in clinical trials?
Phase IV studies are conducted after a therapy has received market approval and gather additional information on long-term risks, benefits, and optimal use in the general population, often involving thousands of participants.
Why is it important to understand the phases of clinical trials?
Understanding these phases is crucial for structuring a clinical study that meets both regulatory standards and scientific objectives, ultimately aiding the successful progression of innovative medical therapies.
What are the key steps in designing a clinical trial?
The key steps include defining the objectives, identifying the target population, choosing the appropriate study design, developing a comprehensive protocol, and estimating budgeting and resources.
How does bioaccess® enhance the clinical trial process?
Bioaccess® facilitates ethical approvals in 4-6 weeks and achieves enrollment 50% quicker than conventional markets, thereby enhancing the effectiveness of clinical studies.