


The article titled "7 ANMAT Compliance Tips for Clinical Research Directors" serves as a crucial resource for clinical research directors aiming to navigate the complexities of ANMAT regulations effectively. It emphasizes the necessity of:
These elements are vital for expediting the approval process and ensuring compliance in the regulatory landscape. By adhering to these guidelines, clinical research directors can enhance their operational efficiency and uphold the highest standards of research integrity.
Navigating the complexities of ANMAT compliance is a critical undertaking for clinical research directors, particularly in a landscape where regulatory efficiency can dictate the success of Medtech innovations. This article presents seven essential tips that empower directors to enhance their understanding and execution of compliance protocols, ultimately leading to more effective clinical studies. As the pressure mounts to deliver timely and successful outcomes, how can directors ensure they are not only meeting regulations but also leveraging best practices to foster innovation and inclusivity in their trials?
bioaccess® leverages its extensive knowledge and local advantages to accelerate research in Medtech innovations. By harnessing Latin America's swift governance, the Balkans' diverse patient demographics, and Australia's efficient ethical approval processes, bioaccess® facilitates research studies with remarkable agility. This streamlined approach encompasses:
This ensures meticulous management of all clinical research aspects. Notably, Colombia offers competitive advantages such as cost efficiency, rapid approvals, high-quality healthcare, and robust patient recruitment capabilities, positioning it as an ideal site for first-in-human trials. Industry leaders emphasize that compliance speed is essential for maintaining competitiveness in the fast-evolving Medtech landscape, enabling quicker adaptation to market needs and patient demands. In 2025, bioaccess® remains at the forefront of medical advancements, exemplifying how regulatory efficiency can significantly influence Medtech innovation and ultimately enhance healthcare delivery. To further illustrate this, case studies, including the partnership with Welwaze Medical Inc. for the Celbrea® launch, alongside endorsements from industry leaders, underscore bioaccess's pivotal role in facilitating successful clinical evaluations.
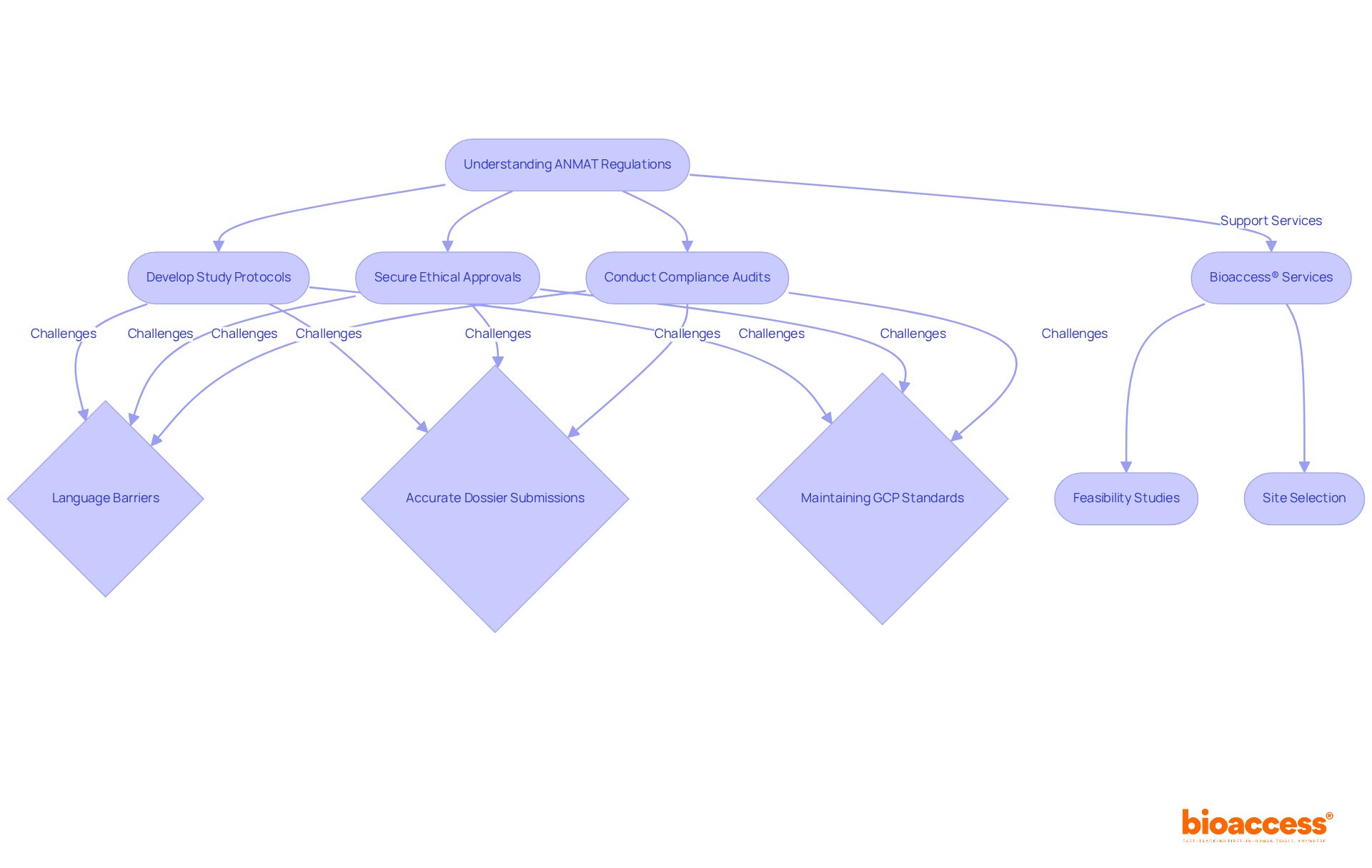
Navigating ANMAT regulations requires a comprehensive understanding of the ANMAT guidelines set forth by the National Administration of Drugs, Food and Medical Technology. Clinical study directors must prioritize their familiarity with essential requirements, which include:
Staying informed about compliance updates is crucial, as is early engagement with ANMAT during the study design phase, which can significantly expedite the ANMAT approval process. Common compliance challenges faced by directors encompass:
By establishing robust compliance frameworks and fostering proactive communication with ANMAT, research directors can enhance their ability to navigate these regulations effectively, thereby ensuring successful study outcomes with ANMAT. As emphasized by the Bioaccess Content Team, "Adopting a collaborative strategy with platforms such as bioaccess® and committing to continuous team education will not only promote regulatory compliance but also guarantee that studies are representative and inclusive."
Understanding that typical timelines for ANMAT approval range from 12 to 18 months highlights the urgency of maintaining compliance. Bioaccess® offers extensive trial management services, including:
These services are essential for navigating the complexities of the Latin American Medtech landscape.
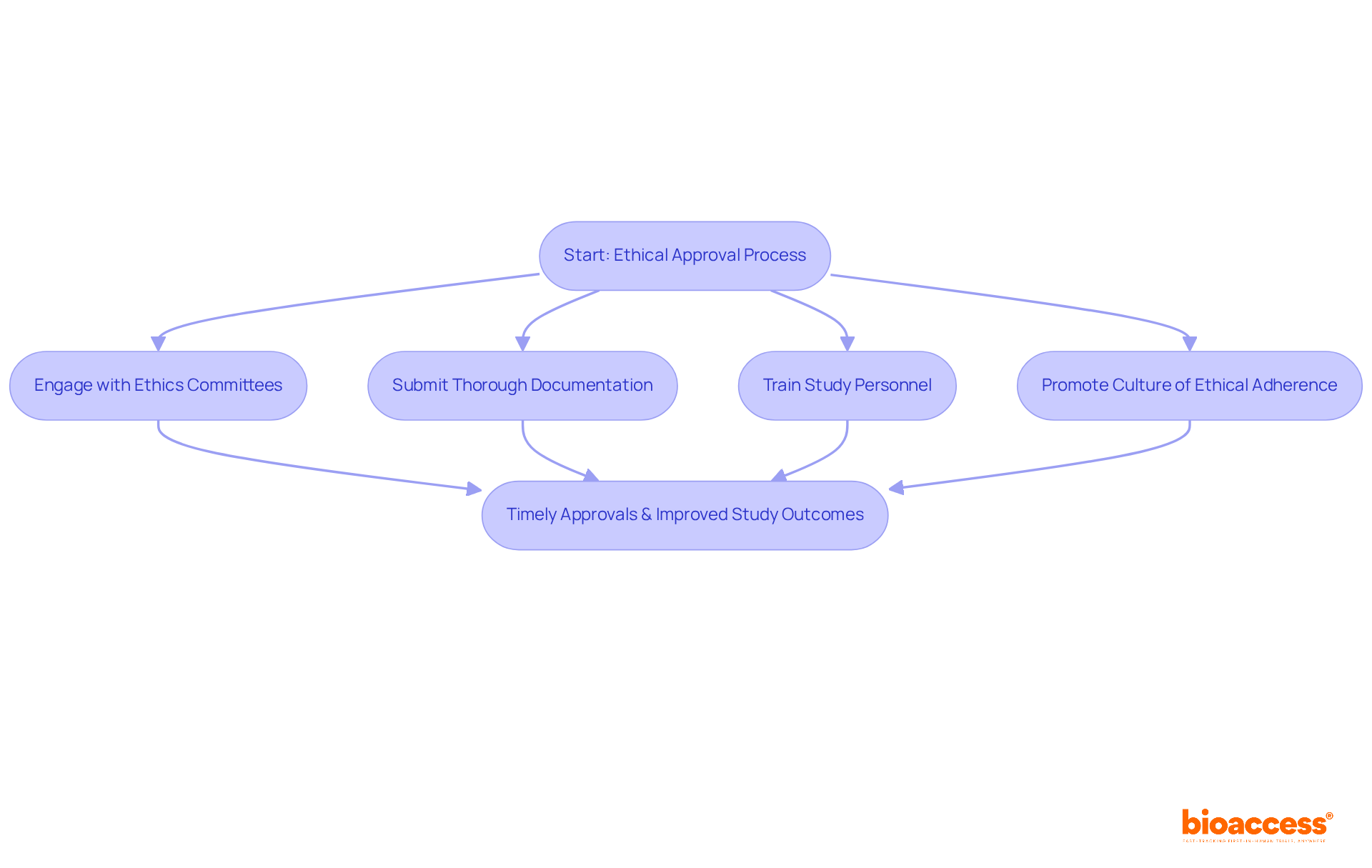
Obtaining ethical approvals is a fundamental aspect of the clinical investigation process that cannot be overlooked. Clinical study directors must prioritize adherence to ethical guidelines, which encompass informed consent and participant safety. Engaging with ethics committees at the outset and submitting thorough documentation can significantly streamline the approval process, enhancing efficiency.
Consistent training for study personnel on ethical standards is essential for upholding integrity in investigations. As emphasized by ethics committee members, promoting a culture of ethical adherence not only boosts the credibility of the study but also positively impacts trial success rates.
Implementing successful strategies, such as early communication with ethics boards and meticulous adherence to submission guidelines, can further facilitate timely approvals, ultimately benefiting study outcomes.
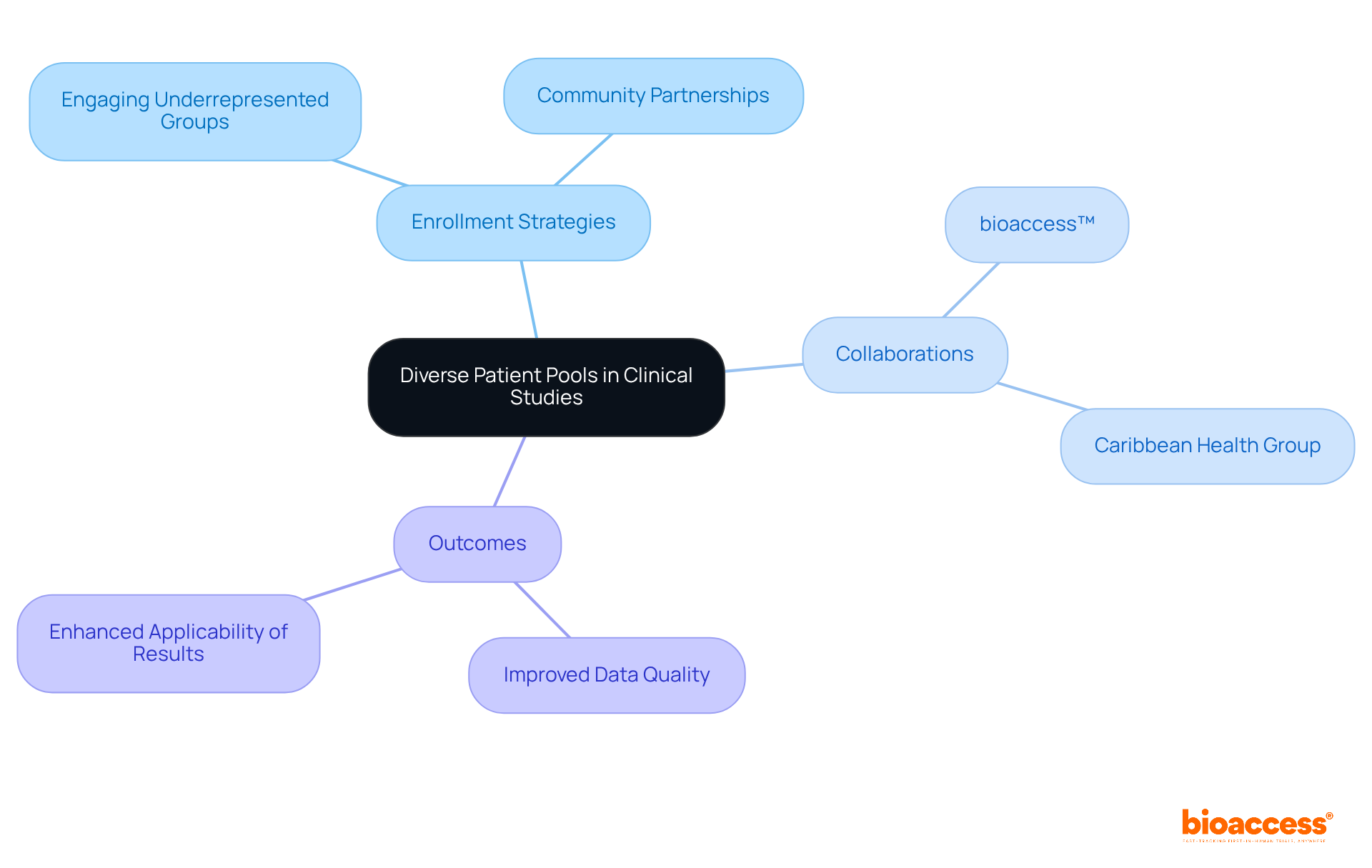
Utilizing diverse patient pools significantly enhances the quality and applicability of clinical study data. Clinical study directors must prioritize enrollment strategies that actively engage underrepresented groups, ensuring that results reflect the broader patient community.
Collaborating with local healthcare providers and community organizations—exemplified by bioaccess™ and Caribbean Health Group's initiative in Barranquilla—can effectively identify and engage diverse participants. This partnership not only facilitates the recruitment of a varied patient demographic but also capitalizes on Colombia's competitive advantages, such as cost efficiency, regulatory speed, and high-quality healthcare.
Ultimately, these efforts lead to more thorough and dependable outcomes.
Early-phase studies, particularly first-in-human (FIH) and early feasibility trials, are crucial in evaluating the safety and efficacy of innovative Medtech solutions. Clinical research directors must prioritize meticulous planning, which includes establishing clear objectives and selecting appropriate endpoints to ensure meaningful outcomes. Strong data-gathering techniques are essential for producing dependable results that can withstand oversight.
Current trends indicate a growing emphasis on the use of biomarkers in FIH studies, significantly enhancing the probability of success. Engaging with governing authorities early in the process is vital for anmat, as it clarifies compliance requirements and facilitates a smoother approval pathway. Bioaccess® exemplifies this proactive approach, obtaining approvals in as little as 6 to 8 weeks, a significant improvement over the usual timelines of 6 to 12 months observed in the US and EU. This acceleration streamlines the approval process and enables the enrollment of treatment-naive cardiology or neurology cohorts at a rate 50% faster than Western sites, addressing common patient recruitment challenges faced by Medtech and biopharma startups.
To achieve these expedited timelines, bioaccess employs a comprehensive strategy that includes early interaction with oversight bodies, careful preparation of submissions, and leveraging local expertise to navigate the complexities of the approval process. For instance, in Avantec Vascular's first-in-human study, bioaccess assisted in selecting a principal investigator and oversaw the submission of the compliance dossier, ensuring a seamless pathway to approval.
Optimal approaches for successful FIH studies in Medtech innovations involve fostering robust communication channels among all parties involved, including sponsors, research sites, and regulatory agencies such as anmat. This partnership is essential for navigating the intricacies of study design and execution. As emphasized by study directors, effective communication and relationship management are critical elements contributing to the success of these trials, ultimately leading to enhanced patient outcomes and market readiness for new therapies.
Creating effective market access strategies is crucial for bridging the divide between scientific studies and commercialization. Research directors must collaborate with market access teams early in the research process to align trial data with market needs. Bioaccess® provides comprehensive market access solutions that simplify the regulatory complexities in Latin America, enabling approvals through anmat that are 40% faster compared to those in the US and EU, facilitating efficient market entry. This includes thorough research study management services such as:
Understanding payer requirements and reimbursement pathways is essential to ensure that innovative products can efficiently reach patients.
Attracting skilled investigators for research studies presents significant challenges, particularly in a competitive landscape marked by limited resources. Clinical study directors can enhance their recruitment efforts by implementing targeted strategies, such as:
Offering competitive compensation packages is essential, as current trends reveal that researcher compensation is evolving to attract top talent. Moreover, cultivating a positive work environment and providing ongoing training opportunities not only draw skilled professionals but also aid in their long-term retention. By prioritizing these strategies, medical study directors can effectively navigate recruitment challenges and assemble a robust team dedicated to advancing clinical trials.
Efficient project management is essential for effectively balancing multiple clinical study initiatives. Clinical study directors must develop comprehensive project plans that explicitly outline timelines, resource allocation, and risk management strategies. Given that nearly 90% of studies struggle to meet recruitment objectives, implementing robust recruitment strategies targeting diverse patient groups is vital to ensure alignment and swiftly address any issues that may arise during the study process.
By leveraging bioaccess®'s specialized expertise and adaptability in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, organizations can significantly enhance recruitment efforts, achieving patient enrollment 50% faster than traditional Western sites and saving $25K per patient with FDA-ready data.
A proactive approach to problem-solving can markedly improve project outcomes, as organizations that embrace structured project management practices waste 28 times less money than those that do not. Furthermore, the integration of technology and data analytics can optimize processes and improve resource distribution, ultimately leading to more effective trials.
As highlighted by industry leaders, maintaining clear communication protocols is critical for fostering collaboration and transparency, ensuring that all stakeholders are aligned with the project's objectives and timelines.
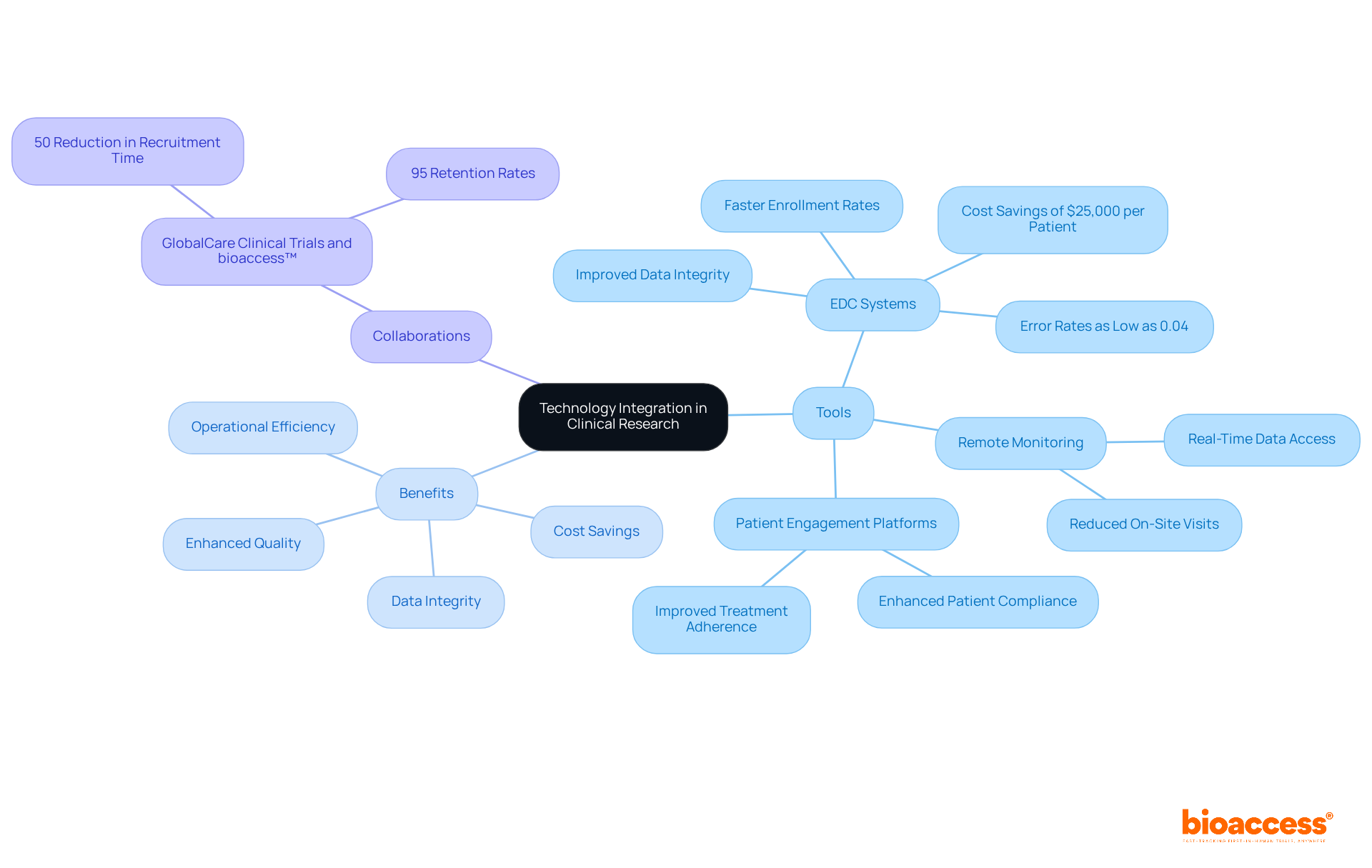
Incorporating technology into clinical study processes significantly enhances operational efficiency and data precision. Clinical study directors must consider the adoption of electronic data capture (EDC) systems, remote monitoring tools, and patient engagement platforms. These digital solutions not only streamline data collection but also alleviate administrative burdens, enabling research teams to concentrate on critical analyses.
For instance, studies utilizing EDC platforms can achieve patient enrollment rates that are 50% faster than traditional methods, resulting in substantial cost savings of $25,000 per patient with FDA-ready data through bioaccess®. Moreover, the implementation of EDC systems has demonstrated improvements in data integrity, with organizations reporting error rates as low as 0.04%. Notably, 70% of EDC users indicate enhanced information quality compared to conventional methods.
Collaborations, such as that between GlobalCare Clinical Trials and bioaccess™, have resulted in a reduction of subject recruitment time by over 50% and an increase in subject retention rates exceeding 95%. With the healthcare data analytics market projected to reach USD 188,305 million by 2033, the importance of leveraging technology in medical studies is paramount. Industry leaders emphasize that timely access to accurate data is essential for effective decision-making, highlighting the transformative potential of digital solutions in improving participant experiences and overall trial outcomes.
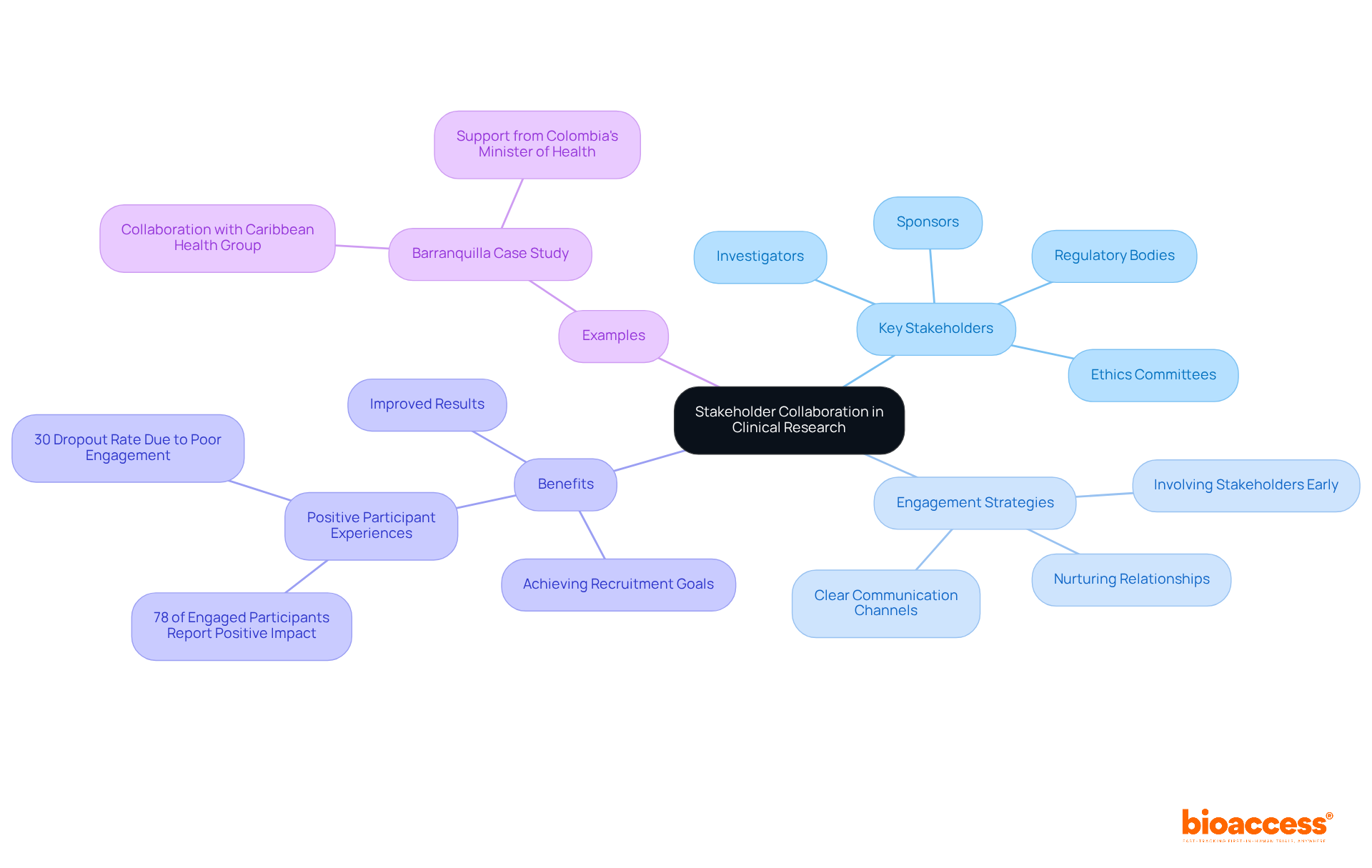
Cooperation among stakeholders is vital for the success of clinical studies. Clinical study directors must prioritize establishing clear communication channels and nurturing relationships with key stakeholders, including:
Involving stakeholders early in the research process not only assists in recognizing potential challenges but also aligns objectives, significantly improving results. For example, bioaccess™ has collaborated with Caribbean Health Group to establish Barranquilla as a prominent location for clinical studies in Latin America, a partnership backed by Colombia's Minister of Health. This initiative exemplifies how strategic partnerships can drive local economic growth and improve healthcare outcomes.
Studies suggest that effective communication strategies can result in enhanced success rates in studies, with organizations that adopt comprehensive engagement methods reaching recruitment goals ahead of time. Notably, 30% of trial volunteers ultimately drop out of their studies due to poor engagement strategies, underscoring the critical need for effective communication. As one study director observed, 'Communication is the backbone of effective stakeholder involvement; it transforms inquiry from a transactional process into a collaborative effort.' Additionally, as Dr. Susan Sheridan emphasizes, 'Patient engagement is not a nice-to-have in modern healthcare research—it's an ethical imperative and a scientific necessity.' By encouraging open conversation and actively engaging stakeholders, study directors can establish a more inclusive atmosphere that ultimately enhances the process and the communities it supports. Moreover, studies indicate that 78% of participants who were actively engaged reported a positive effect on their overall experience, emphasizing the tangible advantages of effective communication and engagement strategies. Comprehensive clinical trial management services offered by bioaccess™, including:
further enhance the collaborative efforts necessary for successful clinical research.
Navigating the complexities of ANMAT compliance is essential for clinical research directors who aspire to enhance the efficiency and success of their studies. This article outlines critical strategies that empower directors to streamline their processes, from grasping regulatory requirements to fostering collaboration among stakeholders. By prioritizing compliance and ethical standards, research teams can significantly improve their outcomes and contribute to advancements in Medtech innovations.
Key insights underscore the importance of:
Furthermore, leveraging technology and establishing robust project management practices can lead to expedited timelines and enhanced data quality. The role of bioaccess® in facilitating these processes illustrates how strategic partnerships can effectively bridge research and commercialization, ultimately improving healthcare delivery.
As the landscape of clinical research evolves, embracing these compliance tips will not only ensure adherence to regulations but also drive meaningful advancements in patient care. By committing to continuous education, fostering stakeholder collaboration, and integrating innovative technologies, clinical research directors can position their studies for success in an increasingly competitive environment. The future of Medtech innovation hinges on the ability to navigate these challenges effectively, making the insights shared in this article invaluable for those at the forefront of clinical research.
What is bioaccess® and how does it contribute to clinical research in Medtech innovations?
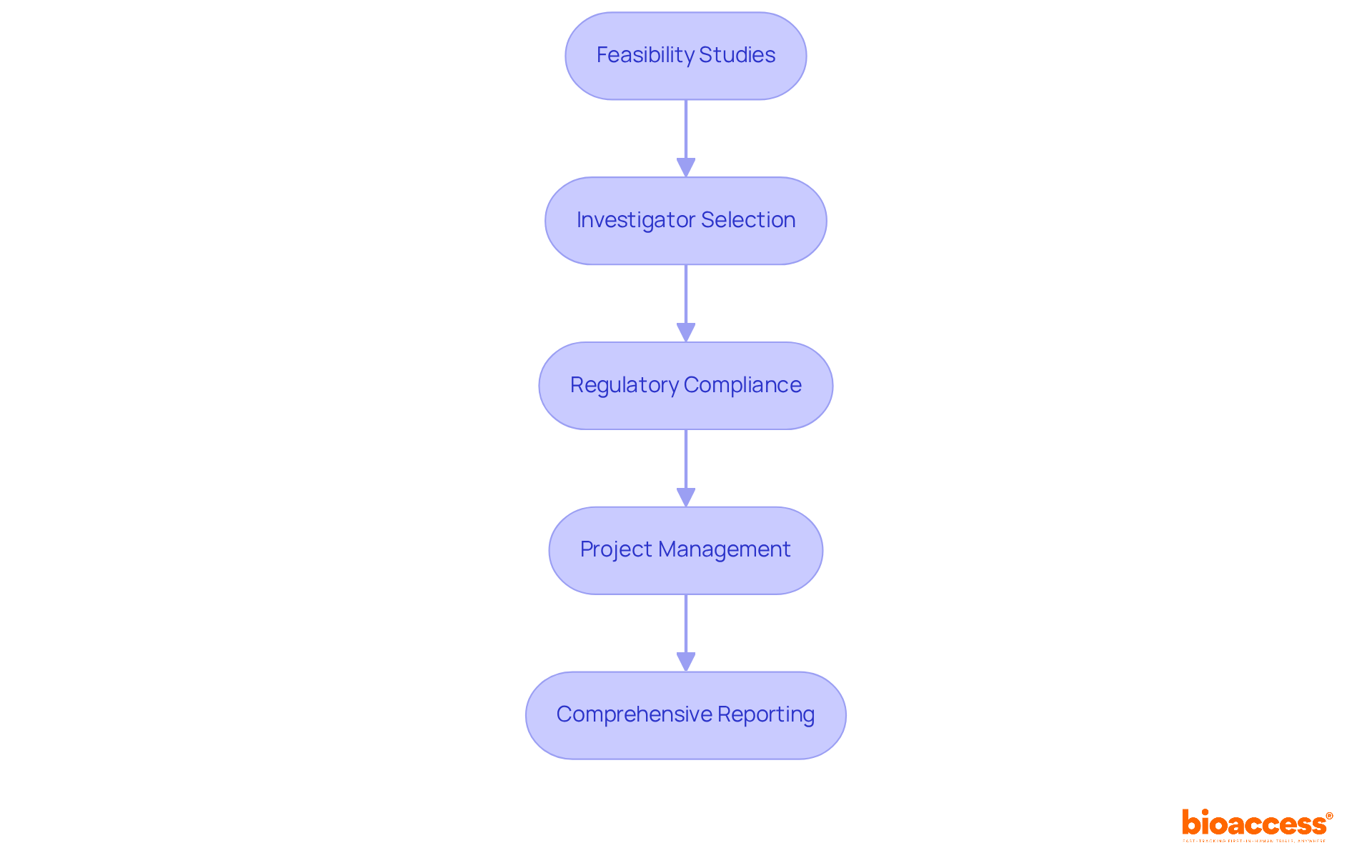
Bioaccess® accelerates clinical research in Medtech innovations by leveraging local advantages in Latin America, the Balkans, and Australia. It facilitates research studies through feasibility studies, investigator selection, regulatory compliance, project management, and comprehensive reporting.
What advantages does Colombia offer for clinical trials?
Colombia provides competitive advantages such as cost efficiency, rapid approvals, high-quality healthcare, and robust patient recruitment capabilities, making it an ideal site for first-in-human trials.
Why is compliance speed important in the Medtech landscape?
Compliance speed is essential for maintaining competitiveness in the fast-evolving Medtech landscape, allowing companies to adapt quickly to market needs and patient demands.
What are the key requirements for navigating ANMAT regulations?
Key requirements include formulating detailed study protocols, securing ethical approvals, and conducting regular compliance audits.
What challenges do clinical study directors face when navigating ANMAT regulations?
Common challenges include language barriers (requiring all documents in Spanish), the need for accurate dossier submissions, and maintaining Good Clinical Practice (GCP) standards.
How can research directors enhance their ability to navigate ANMAT regulations?
By establishing robust compliance frameworks, fostering proactive communication with ANMAT, and staying informed about compliance updates, research directors can improve their navigation of these regulations.
What is the typical timeline for ANMAT approval?
The typical timeline for ANMAT approval ranges from 12 to 18 months.
What role do ethical approvals play in clinical research?
Ethical approvals are fundamental to ensuring participant safety and adherence to ethical guidelines, including informed consent.
What strategies can enhance the ethical approval process?
Strategies include early communication with ethics committees, submitting thorough documentation, and consistent training for study personnel on ethical standards.
How does bioaccess® support trial management services?
Bioaccess® offers extensive trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, which are essential for navigating the Latin American Medtech landscape.