


This article emphasizes the critical task of identifying the leading Contract Research Organizations (CROs) for conducting medical device trials in Mexico. It showcases several prominent CROs, including bioaccess, Medpace, and NAMSA, and underscores their strengths in:
These elements collectively enhance the efficiency and success of clinical trials within the region, making it imperative for stakeholders in the Medtech landscape to recognize the value these organizations bring to overcoming key challenges. Collaboration with these CROs is essential for advancing clinical research and achieving favorable outcomes.
In the rapidly evolving landscape of medical device trials, selecting the right clinical research organization (CRO) can significantly impact the success and speed of bringing innovative products to market. Mexico has emerged as a key player in the global clinical research arena, making it crucial for companies to understand the top CROs available as they navigate the complexities of regulatory compliance and patient engagement.
How can organizations ensure they partner with the most effective CROs that not only meet regulatory demands but also enhance trial efficiency and patient involvement?
This article explores the seven best CROs for device trials in Mexico, highlighting their unique strengths and the value they bring to the research process.
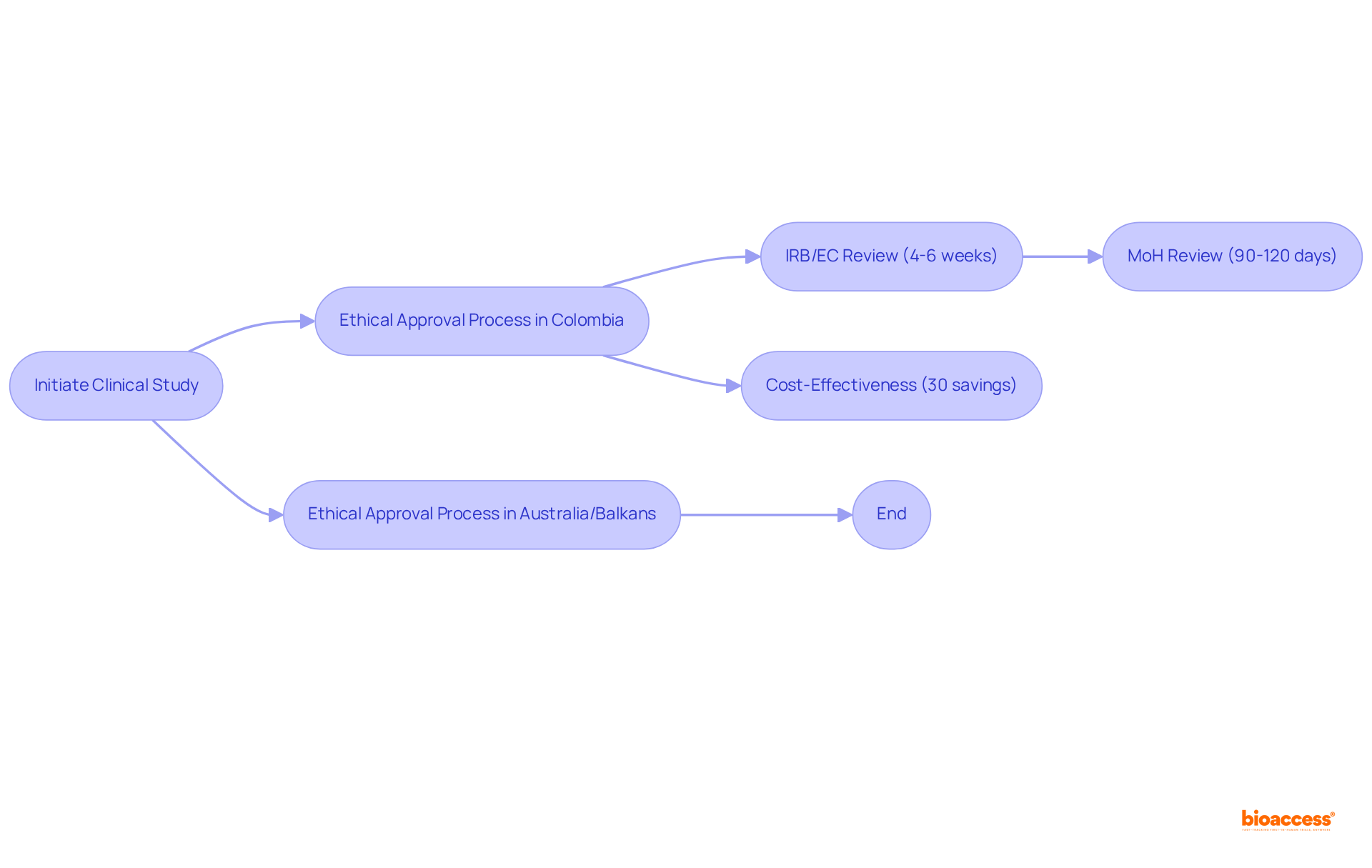
bioaccess® distinctly positions itself within the CRO landscape by delivering rapid ethical approvals, typically within 4-6 weeks. This remarkable speed stems from its profound understanding of Latin America's regulatory environment, particularly in Colombia, where the total IRB/EC and MoH (INVIMA) review process takes merely 90-120 days.
By capitalizing on Colombia's cost-effectiveness—yielding savings exceeding 30% compared to North America and Western Europe—bioaccess® empowers Medtech, Biopharma, and Radiopharma firms to initiate research studies more swiftly than in traditional markets.
Furthermore, with a population surpassing 50 million and 95% coverage under universal healthcare, Colombia offers a diverse patient pool for recruitment. By also leveraging streamlined pathways in Australia and the Balkans, bioaccess® guarantees that clinical research is not only expedited but also adheres to ethical standards, establishing itself as an ideal partner for innovators eager to accelerate their research timelines.
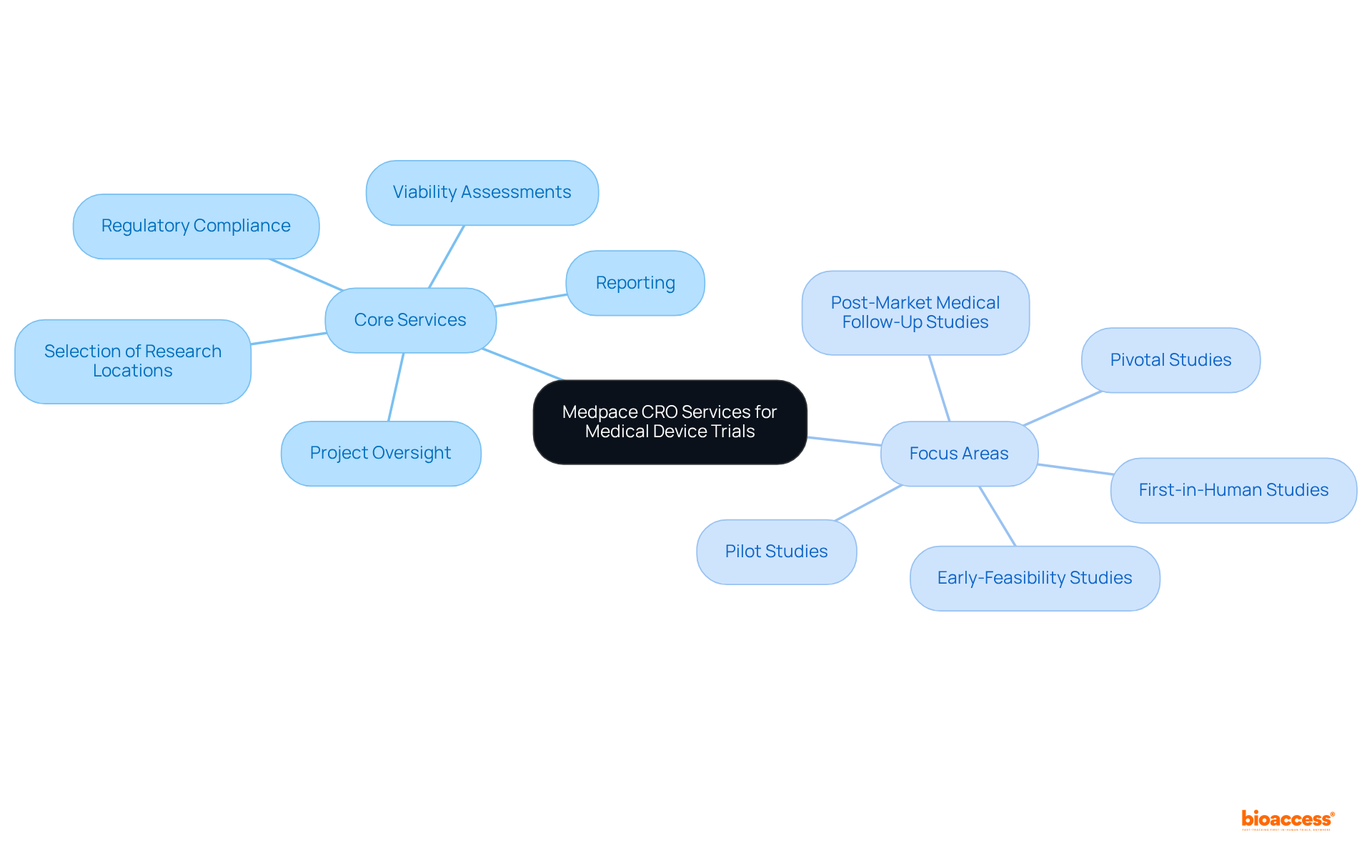
The organization offers a comprehensive suite of services designed to advance medical device studies from inception to completion. Their expertise encompasses:
This ensures that trials are executed with efficiency. With over 20 years of experience in Medtech, this company excels at navigating the intricate regulatory landscapes of Latin America, establishing itself as a dependable partner for organizations aiming to introduce innovative medical devices to the market. Their focus on:
positions them as a leader in the field. To learn more about how bioaccess can support your research needs, schedule a meeting today.
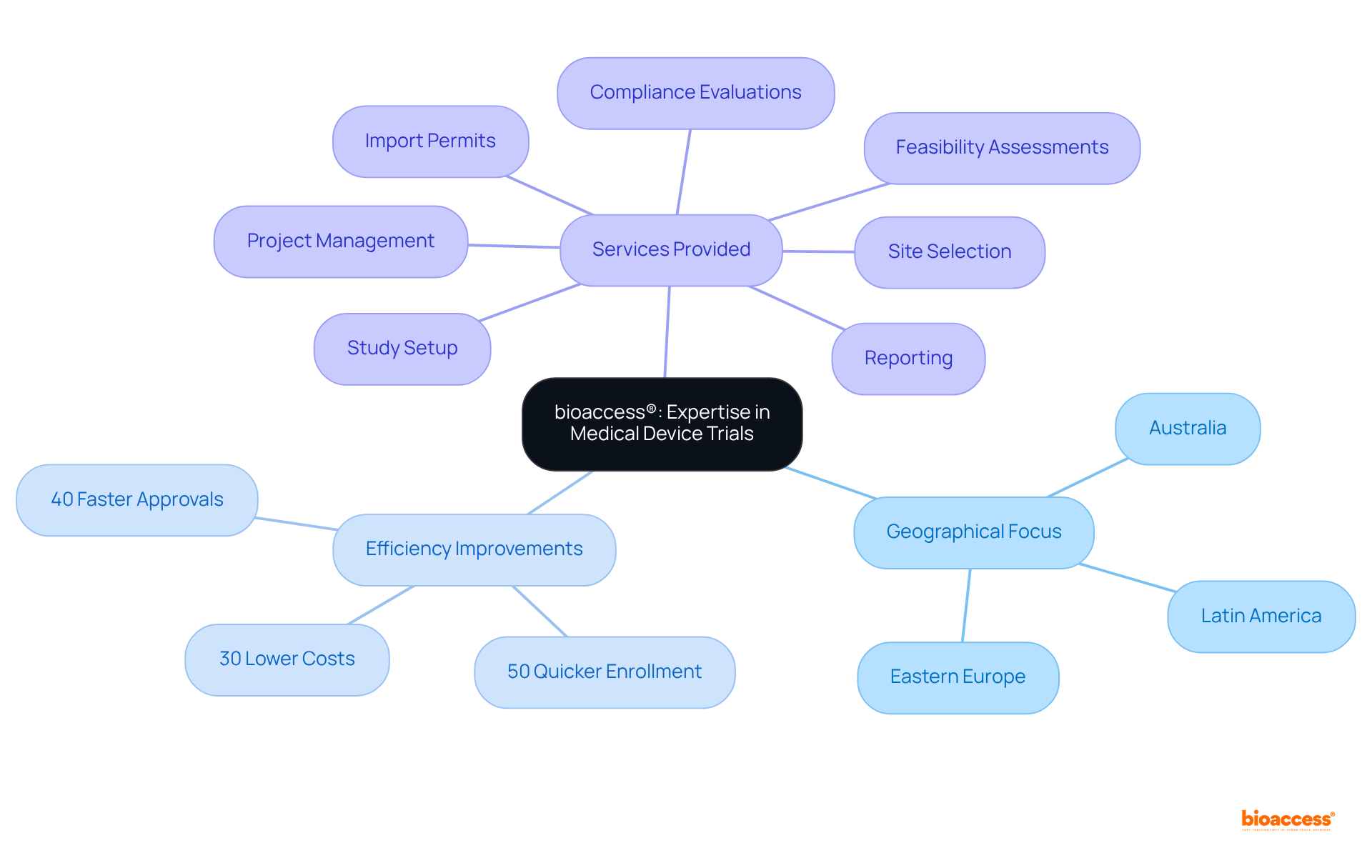
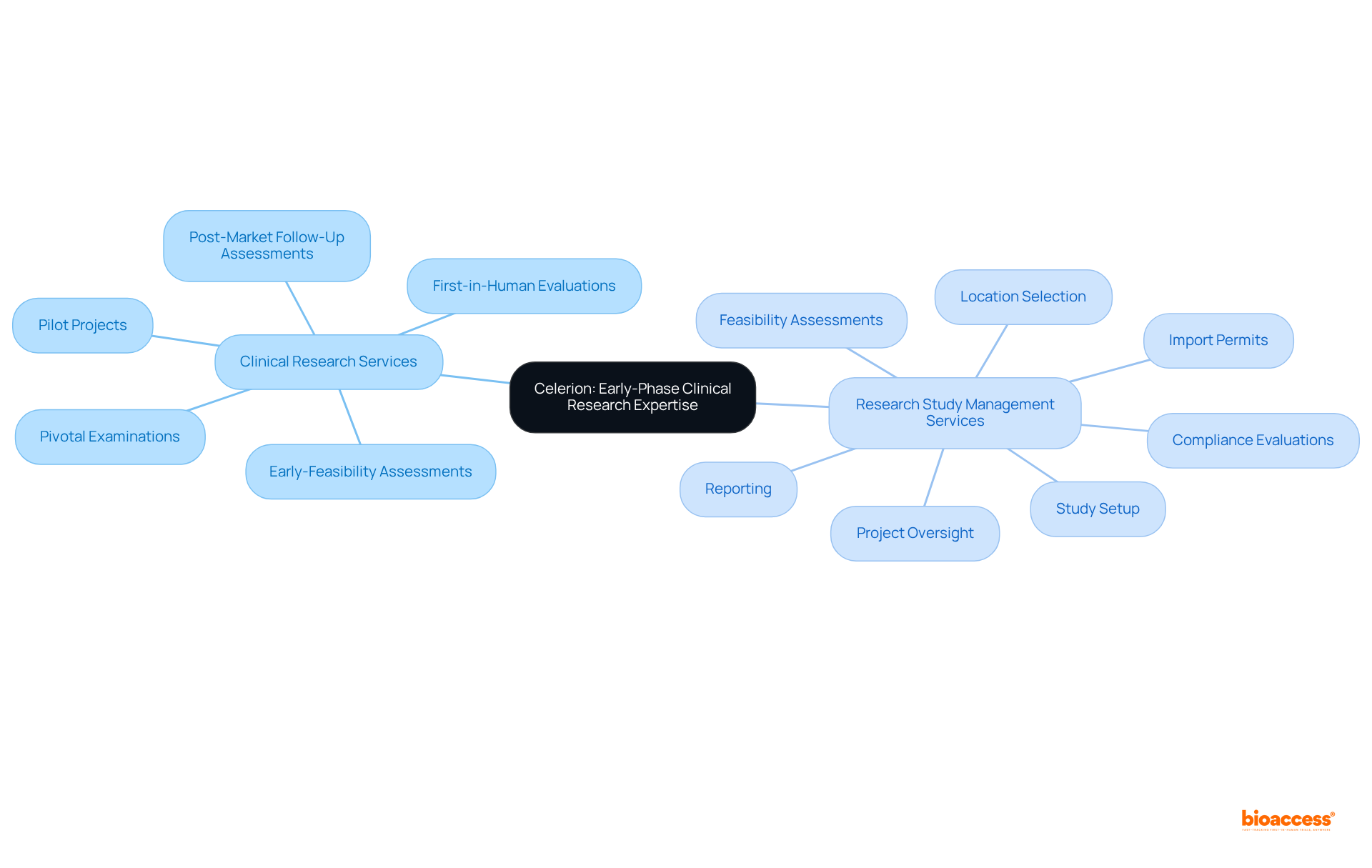
bioaccess® is recognized for its innovative approach to conducting medical device studies, particularly across Latin America, Eastern Europe, and Australia. By prioritizing the acceleration of initial feasibility evaluations and first-in-human experiments, bioaccess® empowers Medtech, Biopharma, and Radiopharma innovators to adeptly navigate the complexities of regulatory demands. Their expertise facilitates approvals that are 40% faster, enrollment that is 50% quicker, and costs that are 30% lower, establishing them as the best cros for device trials in Mexico and making them a strategic partner for companies aiming to penetrate the Mexican market.
Katherine Ruiz, a regulatory affairs expert in medical devices and in vitro diagnostics in Colombia, embodies the extensive knowledge within bioaccess®. Their comprehensive research study management services include:
As bioaccess® articulates, "Our mission is to benefit humanity as a whole," underscoring their dedication to enhancing global health outcomes. This strategic approach not only elevates the efficacy of medical studies but also aligns with the evolving demands of the healthcare market. Engage with bioaccess® today to discover how we can bolster your clinical research initiatives.
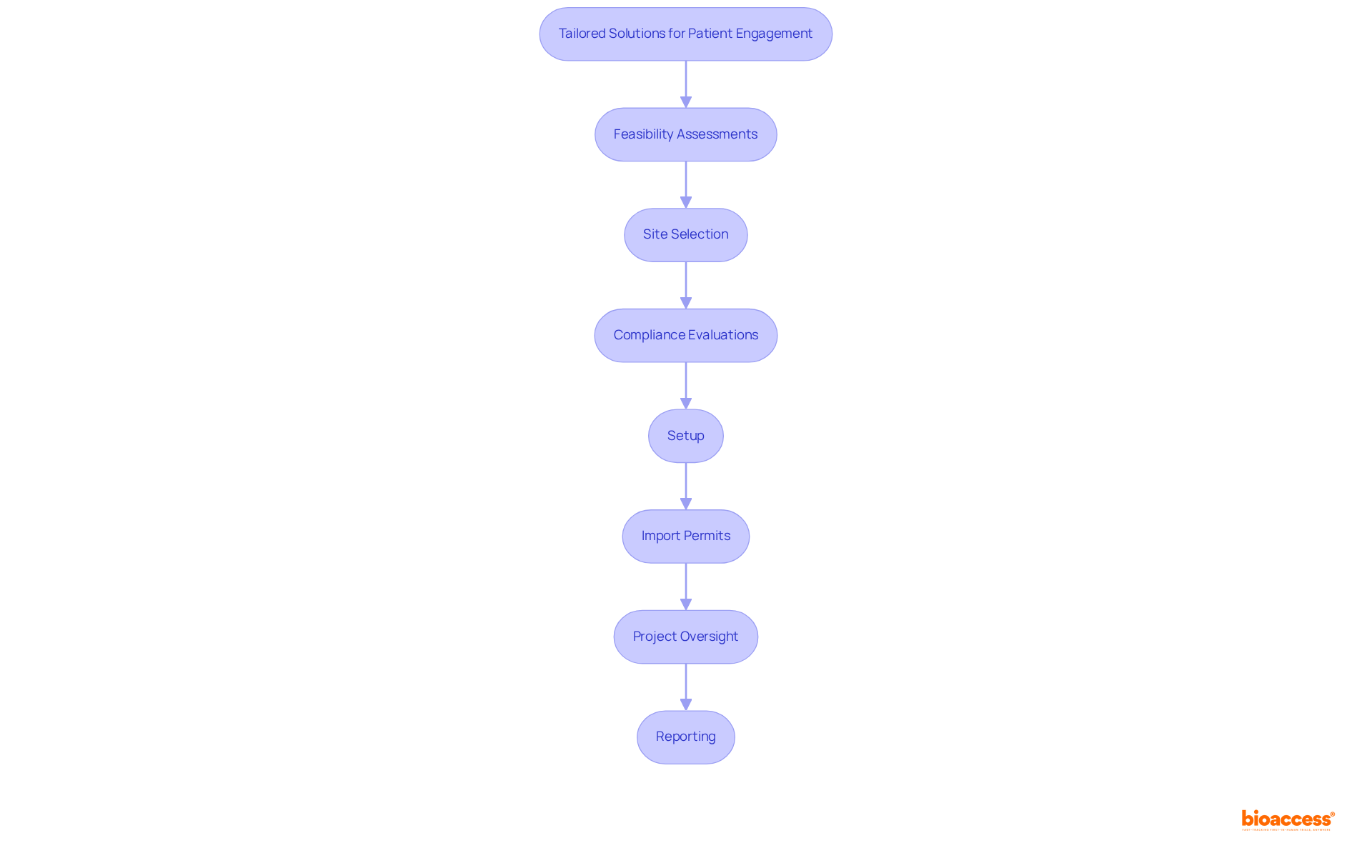
The company excels in enhancing patient involvement through tailored solutions that specifically address the unique requirements of study participants. Their accelerated patient recruitment services leverage a pre-qualified network of over 50 activated sites, ensuring rapid activation in less than eight weeks. By providing FDA/EMA/MDR-compliant datasets and centralized monitoring, the platform significantly improves communication and greatly enhances participant retention rates. This patient-focused approach not only guarantees the effectiveness of studies but also elevates the quality of data collected.
Furthermore, the organization offers comprehensive management services for studies, including:
By prioritizing effective communication and support services, the organization mitigates barriers to enrollment and retention, ultimately resulting in more successful trial outcomes. To learn more about how our solutions can optimize your research results, consider scheduling a meeting with our team.
This specialized CRO, recognized as the best CROs for device trials in Mexico, focuses exclusively on medical device research in Latin America and offers unparalleled expertise in navigating regulatory pathways, including FDA and CE mark approvals. The company provides comprehensive services that encompass:
Their profound understanding of the medical device landscape ensures that clients receive expert guidance throughout the entire product lifecycle, facilitating successful market entry. With over 20 years of experience, this company excels in managing:
This effectively addresses the unique challenges faced by medical device startups in clinical research.
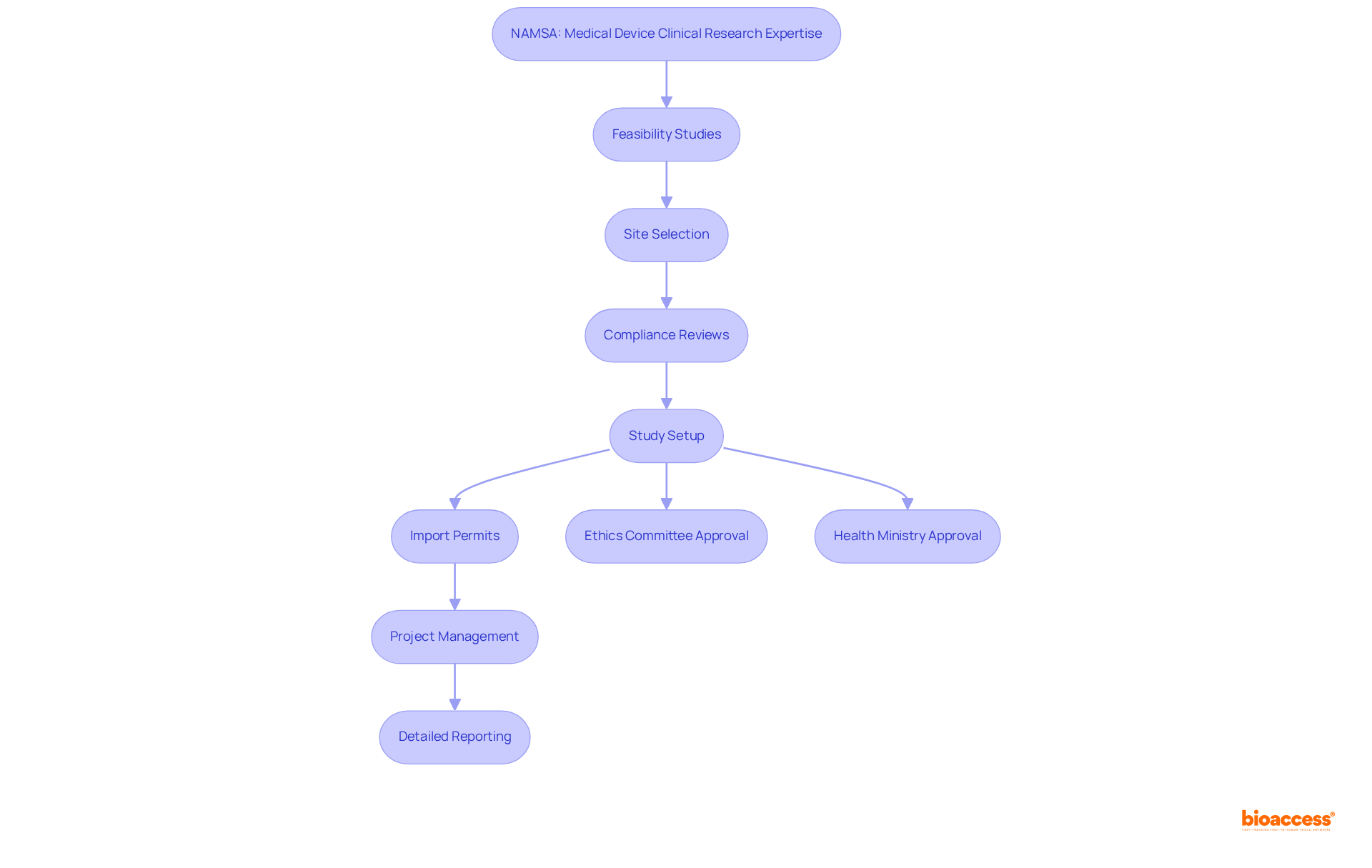
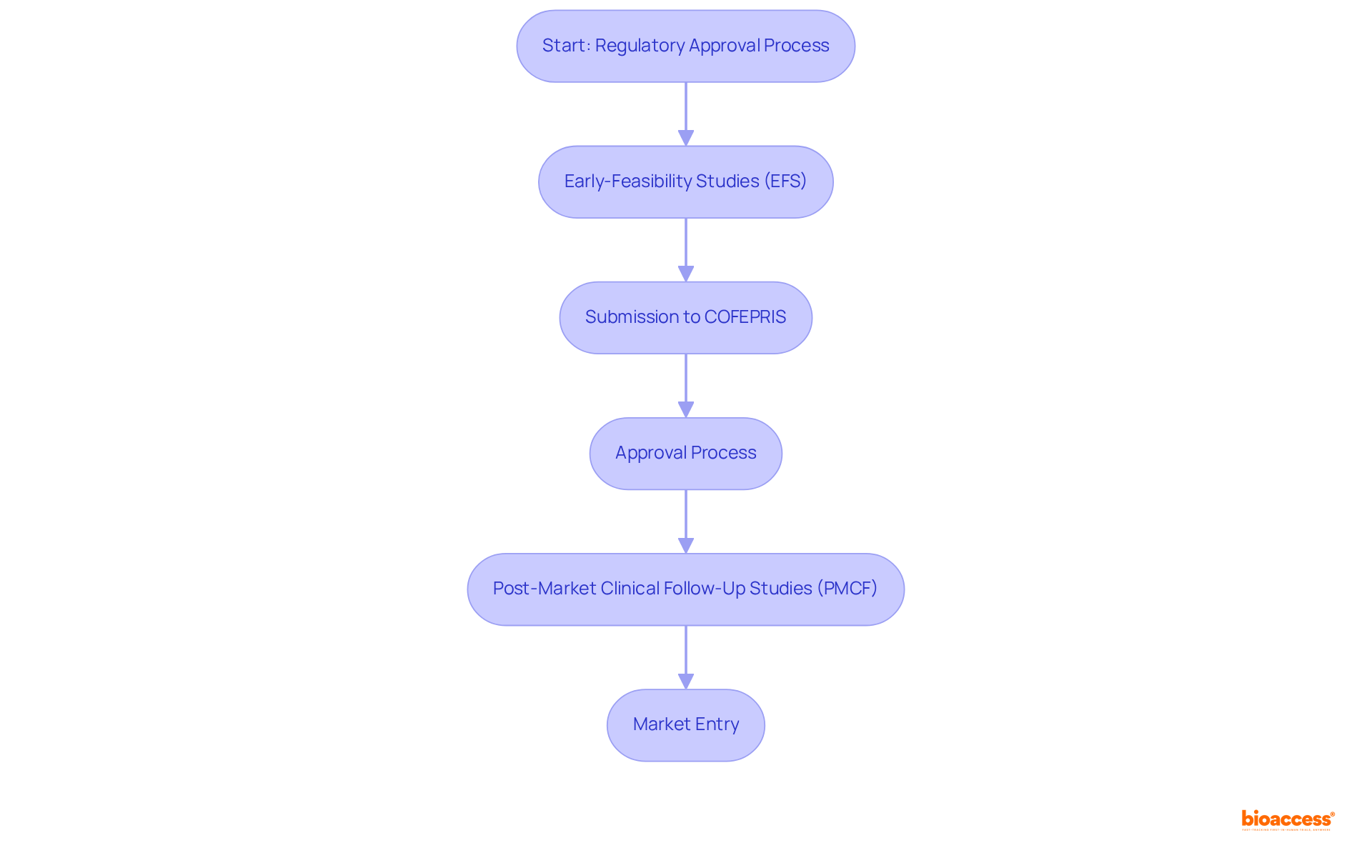
This entity distinguishes itself in the complex regulatory landscape governing medicinal product approvals in Mexico. With a dedicated team of experts who possess over 20 years of experience in Medtech, they provide comprehensive support ranging from Early-Feasibility Studies (EFS) to Post-Market Clinical Follow-Up Studies (PMCF), ensuring strict adherence to local regulations. Their profound understanding of COFEPRIS processes enables them to effectively streamline approval timelines, significantly benefiting clients eager to introduce their products to the Mexican market.
As trends shift towards expedited approvals in 2025, the demand for comprehensive acceleration services for global studies is becoming increasingly vital for firms aiming to navigate these changes adeptly. Their unwavering commitment to meticulous compliance and precision positions them as an indispensable partner for Medtech, Biopharma, and Radiopharma innovators seeking to capitalize on Mexico's burgeoning healthcare market.
Notably, the company has successfully facilitated numerous COFEPRIS submissions, underscoring their expertise in regulatory navigation. Katherine Ruiz, an authority in regulatory affairs for medical devices and in vitro diagnostics in Colombia, highlights the critical importance of comprehending local regulations to ensure successful market entry.
As the landscape evolves, remaining informed about COFEPRIS regulatory updates for 2025 will be essential for companies striving to maintain compliance and leverage market opportunities.
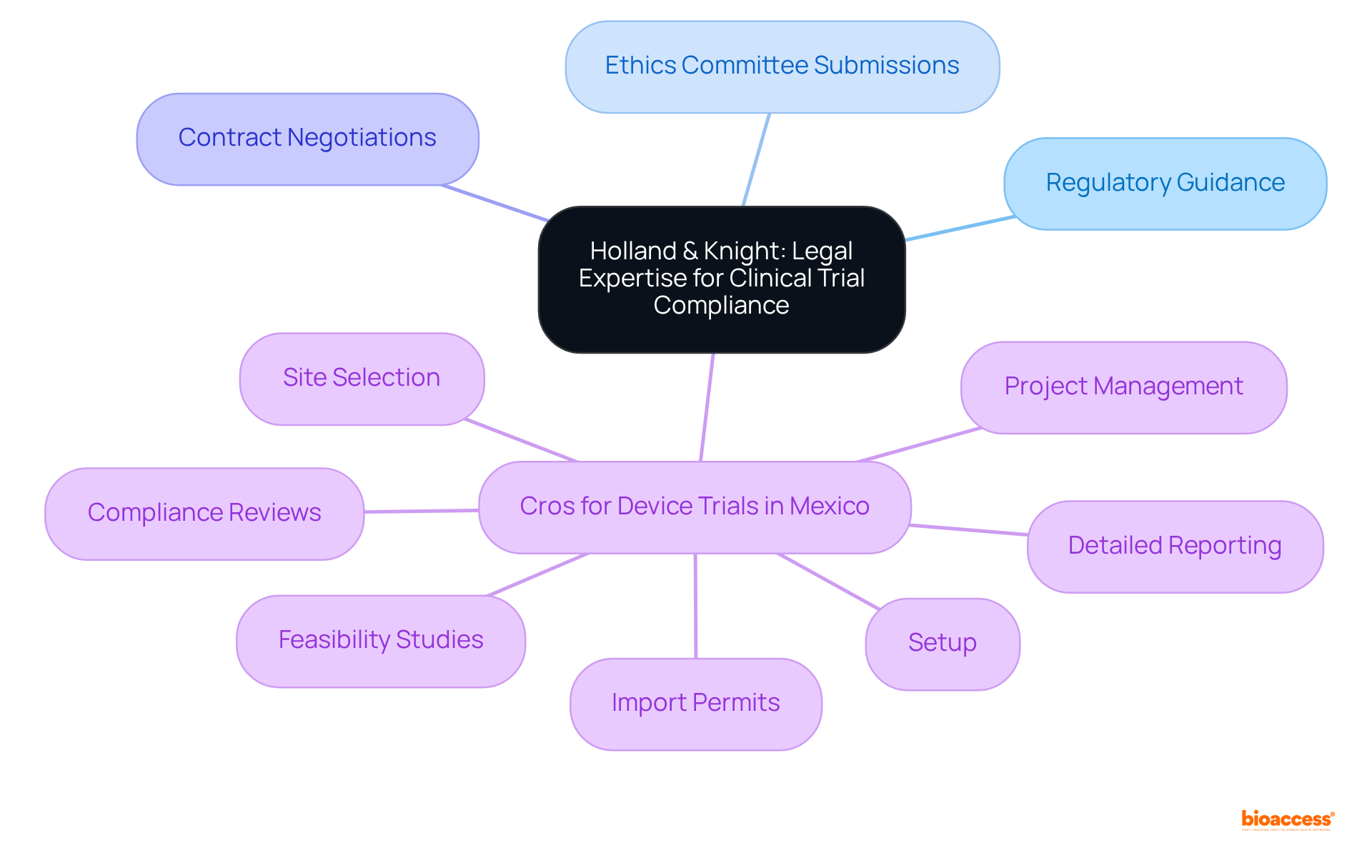
Holland & Knight provides extensive legal assistance for research study compliance, effectively guiding clients through the complex regulatory landscape. Their expertise encompasses advising on FDA regulations, ethics committee submissions, and contract negotiations. In conjunction with legal support, the organization delivers a comprehensive suite of services, including the best cros for device trials Mexico, that facilitate medical device evaluations. This includes:
By meticulously managing all legal and operational aspects of research studies, both Holland & Knight and bioaccess empower clients to mitigate risks and maintain compliance, establishing themselves as trusted partners in the research process.
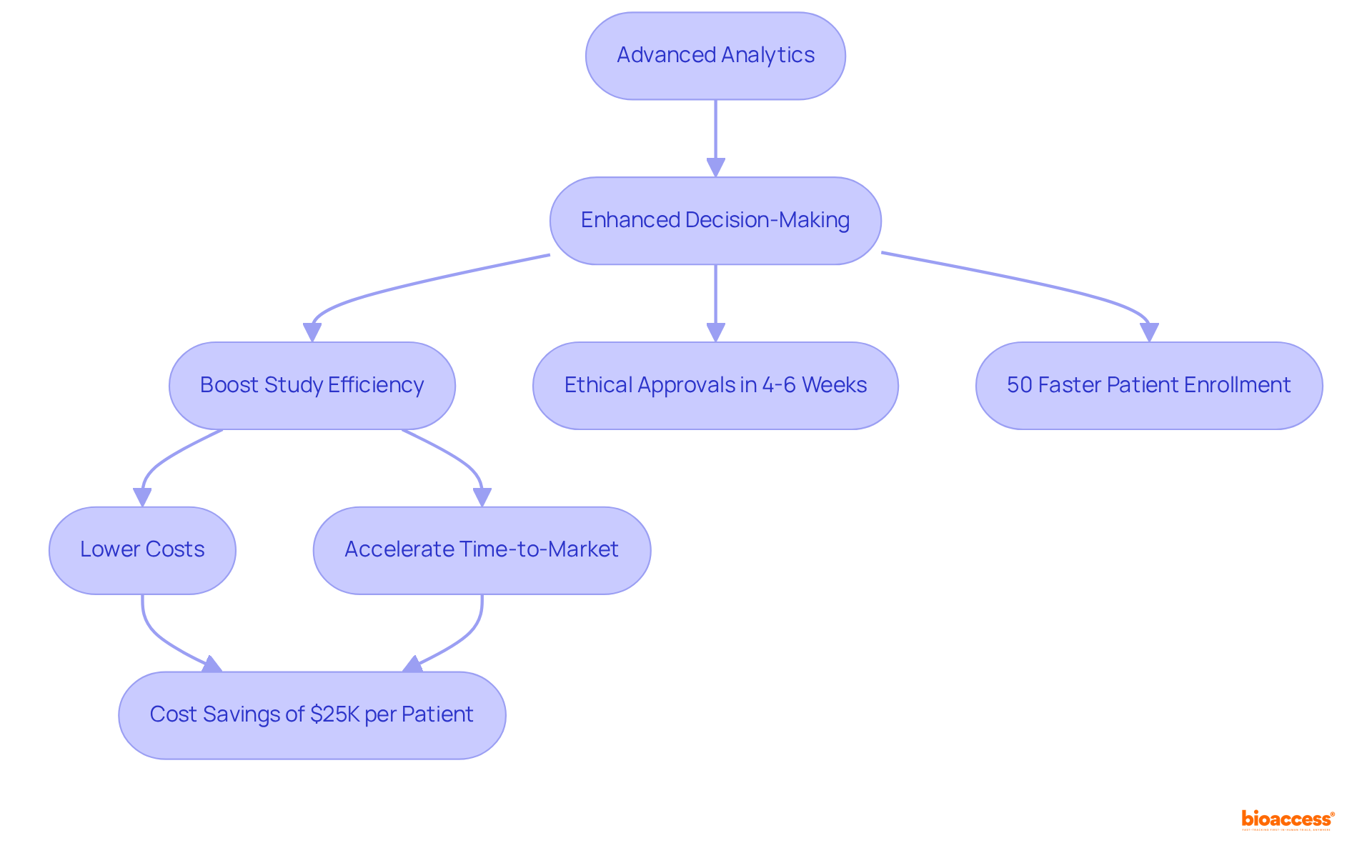
IQVIA harnesses advanced analytics to enhance research processes, providing clients with data-driven insights that significantly improve decision-making. Their innovative solutions, which encompass predictive modeling and real-time data analysis, are instrumental in identifying potential challenges and streamlining operations.
By leveraging IQVIA's analytics capabilities, sponsors can boost study efficiency, lower costs, and accelerate time-to-market for their products. Furthermore, bioaccess® amplifies these efforts by securing ethical approvals in a mere 4-6 weeks and achieving patient enrollment that is 50% faster than traditional markets. This efficiency translates to $25K savings per patient, achieved through strategic site selection and optimized patient recruitment strategies backed by FDA-ready data.
The synergy between cutting-edge analytics and bioaccess®'s regional strengths positions them as the best cros for device trials in Mexico, empowering sponsors to navigate the complexities of research studies in the region with greater efficiency.
The company provides integrated solutions that streamline research studies from planning to execution. Their comprehensive services encompass:
This ensures that clients receive expert assistance at every stage of the experimentation process. By adopting a holistic strategy, the organization empowers sponsors to navigate the complexities of research, ultimately leading to faster and more effective outcomes.
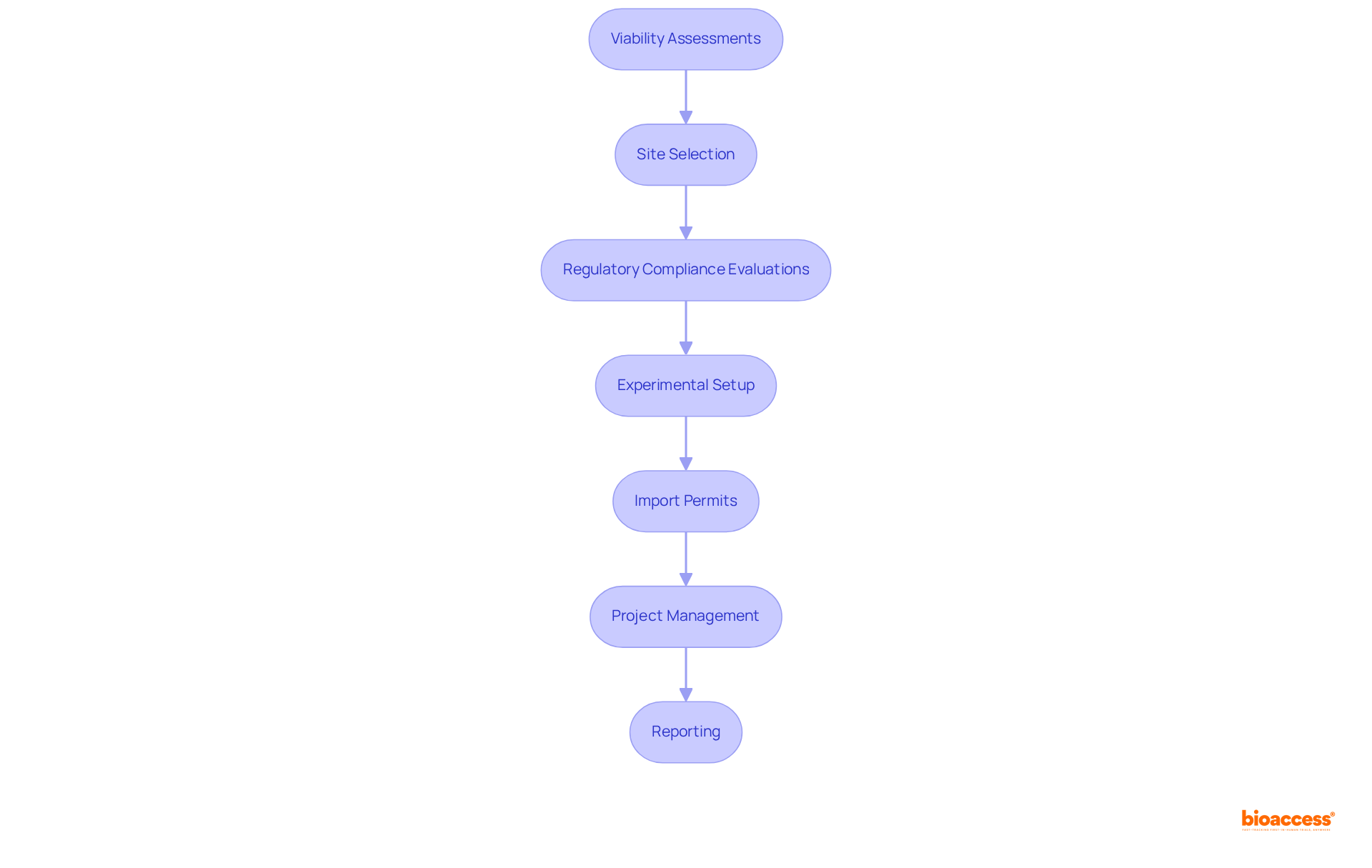
This company stands as a frontrunner in expedited research services, specializing in medical device investigations across Latin America. With over 20 years of experience in Medtech, the company delivers comprehensive solutions that include:
Their expertise guarantees that clients receive FDA/EMA/MDR-ready datasets and efficient site activation, having activated more than 50 pre-qualified sites in under eight weeks. By leveraging extensive research study management services—such as:
clients can adeptly navigate the complexities of regulatory adherence, expediting their market entry strategies. To discover how bioaccess can effectively support your clinical trial needs, contact us today.
The exploration of the best Contract Research Organizations (CROs) for medical device trials in Mexico reveals a landscape brimming with opportunities for innovation and efficiency. Each highlighted CRO, from bioaccess® to Celerion, brings unique strengths that cater to the diverse needs of Medtech, Biopharma, and Radiopharma companies. By leveraging their expertise in regulatory navigation, patient engagement, and accelerated study processes, these organizations position themselves as invaluable partners in the quest to bring groundbreaking medical devices to market.
Key insights from this examination emphasize the importance of speed, compliance, and comprehensive support in clinical research. Companies such as Medpace and NAMSA stand out for their extensive experience and tailored services that ensure trials are executed with precision and adherence to regulatory standards. Meanwhile, organizations like Velocity Clinical and IQVIA highlight the critical role of patient engagement and data analytics in optimizing trial outcomes and enhancing the overall research experience.
As the medical device landscape continues to evolve, the significance of selecting the right CRO cannot be overstated. Companies aiming to capitalize on the burgeoning healthcare market in Mexico must remain informed about the capabilities of these CROs and the regulatory changes on the horizon. Engaging with these expert organizations is essential for navigating the complexities of clinical trials, ensuring compliance, and ultimately accelerating the path to market for innovative medical solutions.
What is bioaccess® and what services does it provide?
bioaccess® is a Contract Research Organization (CRO) that specializes in accelerating clinical research by delivering rapid ethical approvals, typically within 4-6 weeks. It offers services related to regulatory compliance, project oversight, and comprehensive study management, particularly for Medtech, Biopharma, and Radiopharma firms.
How does bioaccess® ensure rapid ethical approvals?
bioaccess® achieves rapid ethical approvals through its deep understanding of Latin America's regulatory environment, especially in Colombia, where the total review process by the Institutional Review Board (IRB) and the Ministry of Health (MoH) takes only 90-120 days.
What are the cost advantages of using bioaccess®?
By capitalizing on Colombia's cost-effectiveness, bioaccess® provides savings exceeding 30% compared to North America and Western Europe, allowing firms to initiate research studies more swiftly and economically.
What patient recruitment advantages does Colombia offer?
Colombia has a population of over 50 million with 95% coverage under universal healthcare, providing a diverse patient pool for recruitment in clinical studies.
What types of studies does bioaccess® support?
bioaccess® supports various types of studies, including early-feasibility studies, first-in-human studies, pilot studies, pivotal studies, and post-market medical follow-up studies.
How does bioaccess® compare to other CROs in terms of speed and cost?
bioaccess® facilitates approvals that are 40% faster, enrollment that is 50% quicker, and costs that are 30% lower than traditional CROs, making it a leading choice for medical device trials, particularly in Mexico.
Who is Katherine Ruiz and what role does she play at bioaccess®?
Katherine Ruiz is a regulatory affairs expert in medical devices and in vitro diagnostics in Colombia, representing the extensive knowledge and expertise within bioaccess®.
What is the mission of bioaccess®?
bioaccess® articulates its mission as benefiting humanity as a whole, emphasizing its commitment to enhancing global health outcomes through effective clinical research.