


The article highlights seven essential EC certificates that are pivotal for success in clinical research, encompassing certifications for ethical compliance, quality management, and cybersecurity. Notable certifications such as ISO 13485 and Good Clinical Practice (GCP) are critical for ensuring regulatory adherence, enhancing data integrity, and improving overall trial outcomes. These certifications facilitate faster market access and foster trust in research findings, underscoring their significance in the Medtech landscape. As clinical research continues to evolve, the role of these certifications becomes increasingly vital, prompting stakeholders to recognize the importance of compliance and quality in achieving successful outcomes.
The landscape of clinical research is increasingly complex, characterized by rapidly evolving regulatory demands and ethical standards. As organizations strive to navigate these multifaceted challenges, the significance of essential EC certificates becomes paramount for ensuring successful trials and timely market access. This article delves into seven critical certifications that not only enhance credibility but also facilitate smoother processes in clinical research.
How can these certifications transform the way trials are conducted and ultimately impact patient outcomes? By exploring this question, we underscore the vital role these certifications play in fostering innovation and improving patient care.
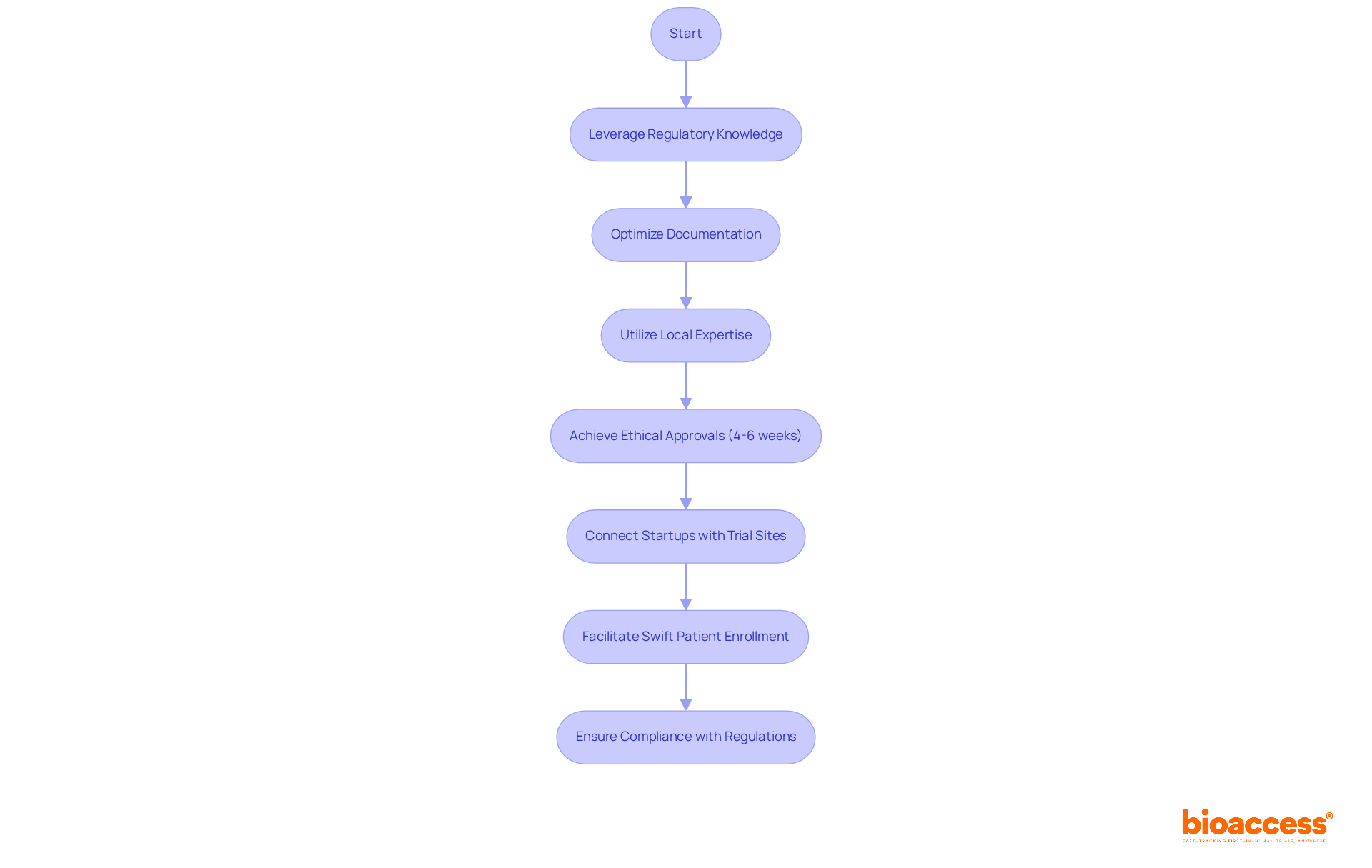
bioaccess® leverages its extensive knowledge of regulatory frameworks across Latin America, the Balkans, and Australia to significantly expedite the EC approval process. By optimizing documentation and harnessing local expertise, bioaccess® achieves ethical approvals in an impressive timeframe of just 4-6 weeks—far surpassing the timelines typical in traditional markets. This accelerated approval is crucial for Medtech, Biopharma, and Radiopharma innovators who aim to swiftly introduce their products to the market. Industry leaders emphasize that fast ethical approvals are vital for maintaining a competitive advantage and ensuring timely access to innovative treatments.
The existing demand for swift ethical approvals underscores the significance of entities like bioaccess® in enabling effective pathways for trials. The typical duration for ethical committee (EC) approval in Medtech and Biopharma has become an essential measure, with bioaccess® establishing a standard that enhances the overall environment for trials. By connecting startups with premier trial sites and facilitating swift patient enrollment, bioaccess® not only accelerates the approval process but also guarantees adherence to FDA, EMA, and MDR regulations, further supporting startups in their medical pursuits.
The EC-Council offers a range of cybersecurity credentials that are vital for maintaining the integrity of medical studies. These qualifications ensure that research data is protected against breaches and unauthorized access, which is essential for safeguarding participant confidentiality and trust. In 2024, the average cost of a healthcare data breach reached $9.8 million, underscoring the financial consequences of insufficient cybersecurity measures.
By implementing robust cybersecurity protocols, organizations can secure their study data, thereby enhancing the overall credibility of their trials. Cybersecurity expert Mohammed Khalil emphasizes, 'Building a resilient defense for the future means returning to these fundamentals with relentless discipline: master MFA, patch your systems, conduct thorough risk analyses, train your people, and hold your vendors accountable.'
This statement underscores the significance of cybersecurity qualifications in mitigating risks associated with data breaches. By prioritizing cybersecurity through EC certificates, clinical research organizations can protect sensitive information, ultimately fostering trust and reliability in their research outcomes.

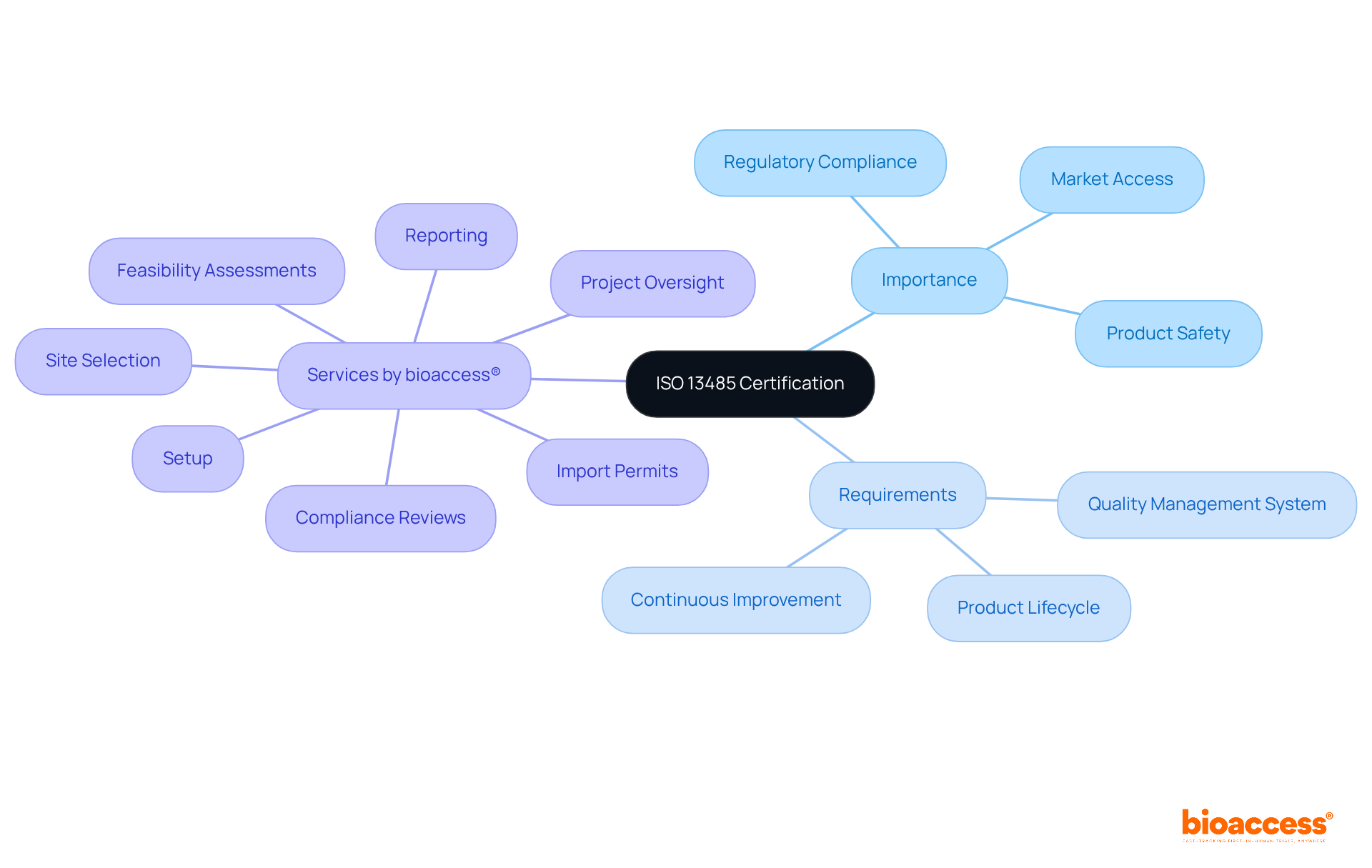
ISO 13485 accreditation stands as a globally recognized standard that outlines the requirements for a quality management system specifically designed for medical devices. This certification is pivotal for organizations striving to consistently meet both customer and regulatory demands, thereby significantly enhancing product safety and efficacy. In the realm of medical studies, ISO 13485 establishes a robust framework for overseeing quality throughout the entire product lifecycle, from initial development to post-market monitoring.
The significance of quality management systems (QMS) in clinical research cannot be overstated. A well-implemented QMS not only ensures compliance with regulatory standards but also cultivates a culture of continuous improvement, which is essential for maintaining high-quality outcomes. For example, a survey of 900 medical device manufacturers revealed that 37% pursued ISO 13485 accreditation primarily to fulfill regulatory requirements, underscoring its critical role in achieving compliance and customer satisfaction.
Recent developments further illuminate the importance of ISO 13485 in the medical device sector. Regulatory experts assert that organizations with ISO 13485 credentials are better positioned to navigate complex legal obligations, thereby enhancing their credibility and market access. As the medical device industry continues to evolve, the adoption of ISO 13485 is increasingly viewed as a strategic advantage, empowering manufacturers to deliver products that consistently meet stringent safety and quality standards.
In conclusion, ISO 13485 certification is not merely a regulatory checkbox; it constitutes a vital component of a successful quality management strategy that directly influences product safety and effectiveness in research. Moreover, for Class III medical devices, which necessitate a notified body for review, ISO 13485 certification is crucial for ensuring compliance and safety, reinforcing its significance across the medical device supply chain. Additionally, bioaccess® offers extensive management services for studies, including:
ensuring that all facets of studies are managed efficiently to meet regulatory standards.
Good Clinical Practice (GCP) represents a crucial global ethical and scientific quality standard for the design, conduct, recording, and reporting of studies involving human subjects. Adhering to GCP is essential for safeguarding the rights, safety, and well-being of research participants, a cornerstone in medical studies. A recent analysis underscored that compliance with GCP significantly impacts study outcomes, with research indicating that adherence can lead to a 20% increase in the likelihood of successful regulatory approval.
Organizations committed to ethical research standards must prioritize GCP training for their personnel. This training not only ensures compliance but also fosters a culture of integrity within medical studies. Industry leaders, including Dr. Beth Garner, emphasize the necessity of diverse participant involvement in research studies to develop effective healthcare solutions. She asserts, "Healthy women should matter to everyone," highlighting the critical need for representation in research.
Moreover, bioaccess has made significant strides in implementing GCP standards through their comprehensive study management services, which include:
This dedication to ethical practices not only protects participants but also bolsters the credibility of the data collected, which is vital for regulatory approval and market access. By prioritizing GCP, bioaccess guarantees that their research studies are conducted ethically and effectively, ultimately leading to improved health outcomes for all while contributing to job creation, economic growth, and healthcare advancement in local communities.

Certification for Clinical Research Associates (CRAs) is essential for ensuring that research studies meet regulatory standards and protocols. Certified CRAs possess the expertise to effectively oversee research sites, verify data integrity, and ensure compliance with Good Clinical Practice (GCP) and other regulatory requirements. This certification not only enhances the credibility of the CRA but also significantly increases the success rates of studies by reducing errors and safeguarding participant safety. In fact, assessments conducted by certified professionals experience fewer errors, resulting in improved patient outcomes and greater job security for these individuals.
As the landscape of medical research evolves, continuous education and updates in regulatory compliance are vital for CRAs to remain effective in their roles. The successful monitoring conducted by certified CRAs has been demonstrated in numerous studies, underscoring their crucial role in maintaining the integrity and reliability of research.

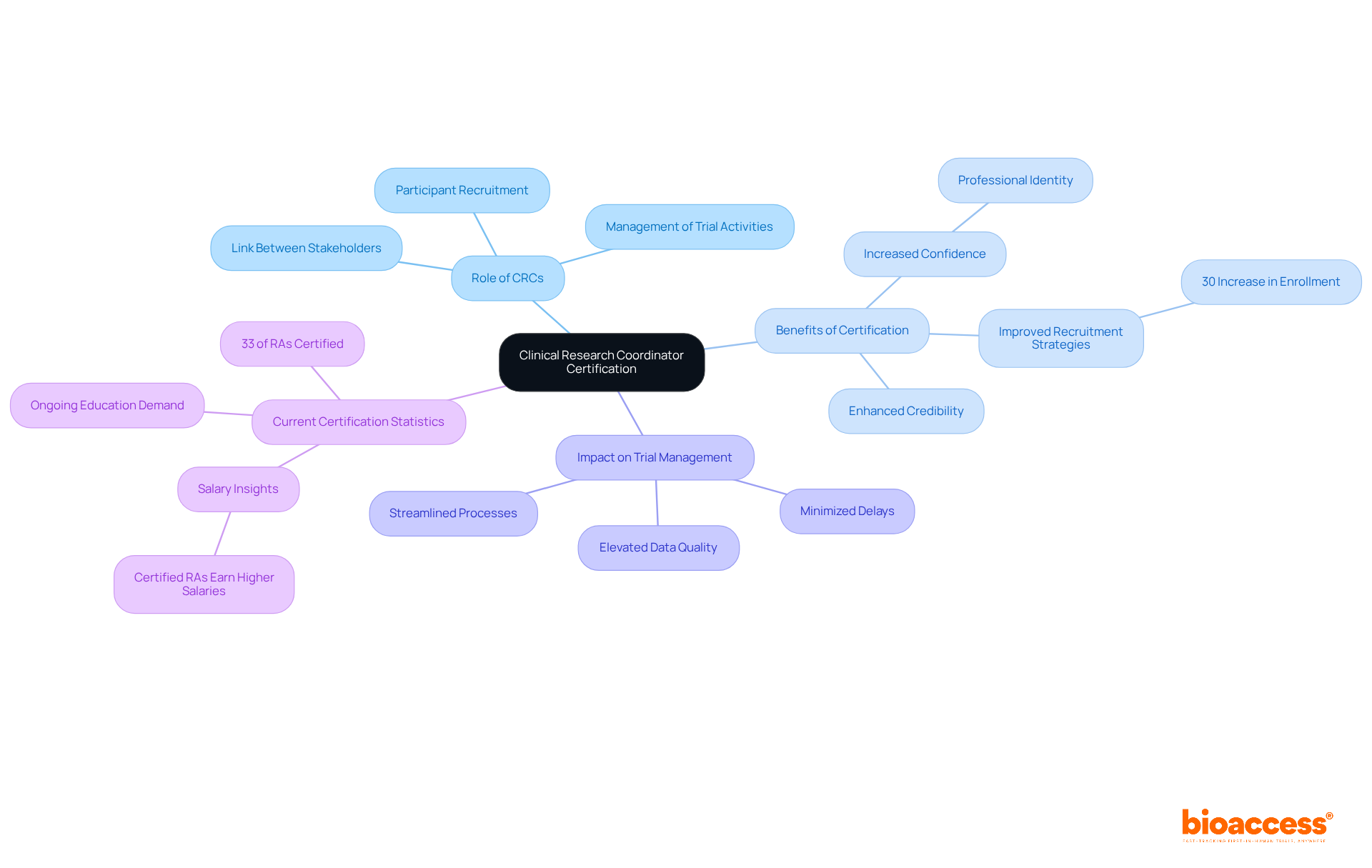
Clinical Research Coordinators (CRCs) are pivotal in the effective management of research studies, acting as the essential link between various stakeholders. Certification for CRCs is vital, as it provides them with the necessary knowledge and skills to proficiently coordinate trial activities, manage participant recruitment, and ensure compliance with regulatory standards. This formal recognition not only enhances the credibility of CRCs but also significantly improves the overall efficiency and effectiveness of research operations.
The significance of CRC training is underscored by industry professionals. One CRC noted, 'Obtaining a credential enhances your confidence,' which illustrates how such qualifications cultivate a sense of professional identity and competence. Furthermore, certified CRCs often demonstrate superior abilities in enhancing participant recruitment and adherence, critical components in the success of clinical studies. For example, a successful case study revealed that a certified CRC implemented targeted recruitment strategies that led to a 30% increase in participant enrollment within a constrained timeline.
The impact of CRC accreditation transcends individual trials; it bolsters trial management efficiency universally. By adhering to best practices and regulatory standards, certified CRCs facilitate streamlined processes, minimize delays, and elevate data quality. As one expert articulated, "Understanding the OPAL calculation provides insights into how integrating longitudinal data on coordinator efforts modifies traditional complexity assessment, justifying OPAL score adaptation and enhancing resource allocation."
As we look towards 2025, the demand for skilled CRCs continues to escalate, emphasizing the necessity for ongoing education and qualifications in this dynamic field. Currently, only 33% of Research Associates (RAs) possess certification, revealing a substantial opportunity for growth in this sector. The commitment to professional development not only benefits CRCs but also elevates the standards of health studies overall. Moreover, with bioaccess's extensive management services for research studies—including feasibility analyses, site selection, and project oversight—CRCs are better equipped to navigate the complexities of these investigations, ultimately contributing to the success of research initiatives and the enhancement of local economies through job creation and advancements in healthcare.
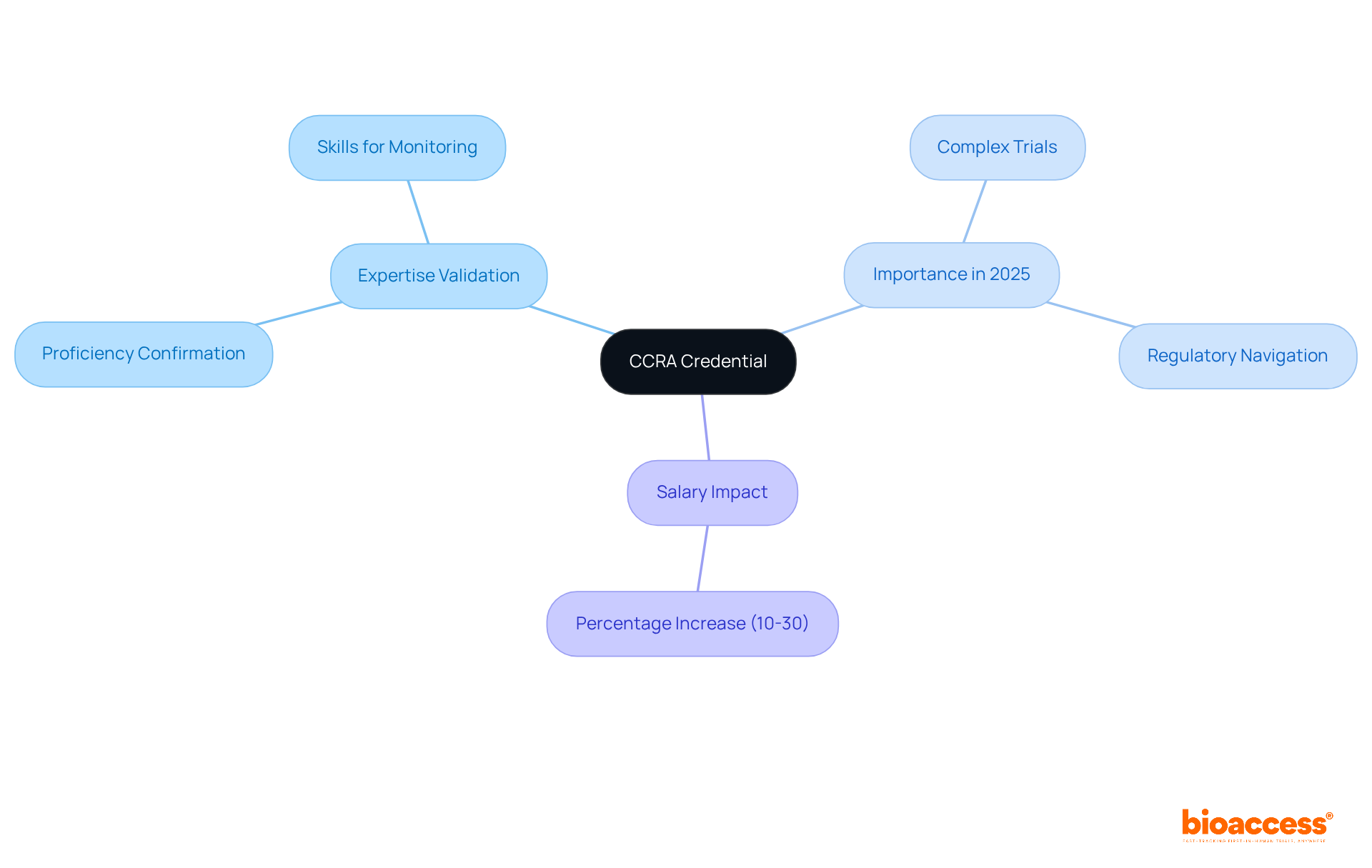
The Certified Clinical Research Associate (CCRA) credential signifies a high level of expertise in the oversight and management of research studies. This accreditation not only confirms the proficiency of Clinical Research Associates (CRAs) but also ensures they possess essential skills for effective study monitoring and regulatory compliance. By obtaining CCRA accreditation, CRAs significantly enhance their professional trustworthiness, a vital component in maintaining the integrity of patient-related research.
In 2025, the importance of CCRA accreditation is underscored by the increasing complexity of research trials, where certified experts are recognized for their adeptness in navigating regulatory landscapes and upholding ethical standards. Notably, research indicates that certified CRAs can earn 10% to 30% more than their non-certified counterparts, emphasizing the value placed on credentials within the industry. As the demand for skilled professionals continues to rise, the CCRA qualification serves as a crucial asset for CRAs aiming to excel in their roles and contribute meaningfully to the advancement of medical studies.
Clinical Research Certification (CRC) stands as a pivotal credential for professionals seeking to elevate their careers in clinical research. This credential not only signifies a commitment to excellence and adherence to industry standards but also enhances the job prospects of certified individuals. According to industry insights, credentials such as the CCRC and CCRP can elevate CRC salaries by $5,000 to $10,000 annually compared to their non-certified counterparts, underscoring the tangible benefits of these qualifications on earning potential.
Experts pursuing CRC credentials frequently report significant advancement in their careers. For example, many CRCs transition into higher-paying roles such as Clinical Research Associate (CRA) or Clinical Trial Manager, often nearly doubling their salaries within five years. As noted, "Many CRCs transition into CRA, Data Manager, or Trial Manager positions, nearly doubling their income within five years." The demand for certified professionals is projected to grow, with the clinical research market anticipated to surpass $80 billion by 2025, creating abundant opportunities for those equipped with the right credentials.
Moreover, credentials are increasingly recognized as a key differentiator in a competitive job market. Employers understand that certified professionals possess essential knowledge of Good Clinical Practice (GCP) and regulatory compliance, rendering them more suitable for leadership roles and complex projects. As one specialist articulated, "Employers regard accreditation as evidence of GCP expertise, regulatory understanding, and preparedness to handle decentralized study systems." As the landscape of medical studies evolves—particularly with the rise of decentralized evaluations—the significance of CRC qualification in enhancing job opportunities and salary potential cannot be overstated. Consider pursuing CRC credentials to bolster your career opportunities and income potential in the expanding field of healthcare research.

The Certified Clinical Research Coordinator (CCRC) credential is awarded to professionals demonstrating expertise in managing medical studies. This vital accreditation ensures that CRCs possess the necessary skills to coordinate study activities, engage with participants, and maintain compliance with regulatory standards. By obtaining CCRC certification, professionals significantly enhance their credibility and effectiveness in research management.
Furthermore, understanding the comprehensive processes involved in advancing medical device studies—such as:
greatly empowers a CRC's ability to navigate the complexities of research. With services like those provided by bioaccess, including feasibility studies, compliance reviews, trial setup, and reporting, CRCs are well-prepared to tackle the challenges faced by medical device startups, particularly regulatory hurdles and financial constraints.
The Medical Device Single Audit Program (MDSAP) offers a transformative solution for medical device manufacturers, allowing them to undergo a single regulatory audit that satisfies the requirements of multiple jurisdictions, including Australia, Brazil, Canada, Japan, and the United States. This program significantly streamlines compliance processes, alleviating the burden of multiple audits and enabling faster market access. By participating in MDSAP, manufacturers can achieve earlier market entry; studies indicate that MDSAP-certified companies can reduce their time-to-market compared to traditional methods, which may take 12-18 months for multi-country regulatory approval.
Leveraging MDSAP enhances operational efficiency and ensures adherence to international regulatory standards, a crucial factor for successful clinical research and product development. The program simplifies documentation requirements and aligns quality management systems with the most stringent regulations across target markets. Consequently, companies can allocate their resources more effectively towards core business activities rather than managing numerous audit processes.
Industry leaders acknowledge the strategic advantages of MDSAP, recognizing it as a quality differentiator in competitive tenders and procurement processes. The comprehensive nature of MDSAP audits identifies and addresses quality system gaps that may be overlooked in single-jurisdiction audits, thereby reducing long-term compliance risks and enhancing overall product integrity. This proactive approach to regulatory compliance positions manufacturers favorably in the global market.

In conclusion, the essential EC certificates outlined in this article are critical components for achieving success in clinical research. These certifications streamline processes and enhance the credibility and integrity of research studies. By prioritizing certifications such as ISO 13485, GCP, and those offered by the EC-Council, organizations can ensure compliance with regulatory standards, protect sensitive data, and ultimately deliver safe and effective medical solutions to the market.
Key arguments highlighted include:
Each certification contributes uniquely to the overall success of clinical research, reinforcing the value of investing in these credentials.
As the landscape of clinical research evolves, the demand for certified professionals will only continue to grow. Organizations and individuals are encouraged to embrace these essential certifications to enhance their operational efficiencies and foster a culture of integrity and trust within the industry. By taking proactive steps toward obtaining these qualifications, stakeholders can significantly impact the success of clinical trials and the advancement of healthcare innovations.
What is bioaccess® and what role does it play in clinical research?
bioaccess® is an organization that leverages its knowledge of regulatory frameworks across various regions to expedite the ethical committee (EC) approval process for clinical research. It achieves ethical approvals in a timeframe of just 4-6 weeks, which is significantly faster than traditional markets, benefiting Medtech, Biopharma, and Radiopharma innovators.
How does bioaccess® enhance the EC approval process?
bioaccess® optimizes documentation and utilizes local expertise to facilitate swift ethical approvals. It connects startups with premier trial sites and ensures adherence to FDA, EMA, and MDR regulations, ultimately supporting startups in their medical pursuits.
Why are fast ethical approvals important in clinical research?
Fast ethical approvals are crucial for maintaining a competitive advantage and ensuring timely access to innovative treatments. The demand for swift approvals highlights the importance of organizations like bioaccess® in creating effective pathways for trials.
What is the EC-Council and what certifications do they offer?
The EC-Council offers a range of cybersecurity credentials essential for maintaining the integrity of medical studies. These certifications help protect research data against breaches and unauthorized access, ensuring participant confidentiality and trust.
What are the financial implications of insufficient cybersecurity in healthcare?
In 2024, the average cost of a healthcare data breach reached $9.8 million, highlighting the financial consequences of inadequate cybersecurity measures for organizations involved in clinical research.
How can organizations enhance the credibility of their trials through cybersecurity?
By implementing robust cybersecurity protocols and obtaining EC certifications, organizations can secure their study data, which enhances the overall credibility and reliability of their research outcomes.
What is ISO 13485 and why is it important for medical devices?
ISO 13485 is a globally recognized standard that outlines the requirements for a quality management system specifically for medical devices. It is essential for organizations to consistently meet customer and regulatory demands, significantly enhancing product safety and efficacy throughout the product lifecycle.
What are the benefits of having a Quality Management System (QMS) in clinical research?
A well-implemented QMS ensures compliance with regulatory standards, fosters a culture of continuous improvement, and is crucial for maintaining high-quality outcomes in clinical research.
Why do organizations pursue ISO 13485 accreditation?
Organizations pursue ISO 13485 accreditation primarily to fulfill regulatory requirements, enhance their credibility, and improve market access, thus ensuring compliance and customer satisfaction.
What services does bioaccess® offer for managing studies?
bioaccess® provides extensive management services including feasibility assessments, site selection, compliance reviews, setup, import permits, project oversight, and reporting to ensure that all facets of studies meet regulatory standards efficiently.