


The article centers on the critical importance of Device Master Records (DMRs) in optimizing clinical research and ensuring regulatory compliance. It asserts that well-maintained DMRs are instrumental in streamlining clinical trial processes, enhancing product quality and safety, and facilitating adherence to regulatory standards. This ultimately leads to improved patient outcomes and accelerates market access for medical products. By addressing these key aspects, the article underscores the essential role DMRs play in the Medtech landscape, positioning them as a foundational element in overcoming challenges faced in clinical research.
Device master records (DMRs) are not merely regulatory necessities; they are pivotal in shaping the efficiency and integrity of clinical research. By consolidating critical documentation, these records streamline the often complex processes of approval and patient enrollment, ultimately enhancing patient outcomes and expediting market access for innovative medical devices.
However, as the landscape of medical technology evolves, a significant challenge persists: how can organizations effectively manage and maintain these essential records to ensure compliance and operational excellence?
This article delves into seven essential insights on device master records, uncovering their significance and best practices for managing them in the realm of clinical research.
At bioaccess®, the integration of device master records is a cornerstone for optimizing clinical research workflows. By centralizing all essential documentation, bioaccess® accelerates both the approval and enrollment phases of clinical trials. This streamlined approach not only enhances adherence to regulatory standards but also significantly reduces the timeline for ethical approvals, facilitating quicker patient recruitment and study initiation.
A well-maintained device master records system is indispensable for safeguarding the integrity and quality of clinical research, ultimately enhancing patient outcomes and expediting market access for innovative medical products. Furthermore, the device master records serve as a comprehensive resource that supports training for new staff, ensuring consistency and adherence to established protocols.
With effective device master records management, organizations can minimize manufacturing mistakes, enhance operational efficiency, and uphold a robust structure for regulatory compliance, thereby reinforcing their commitment to producing safe and effective medical products.

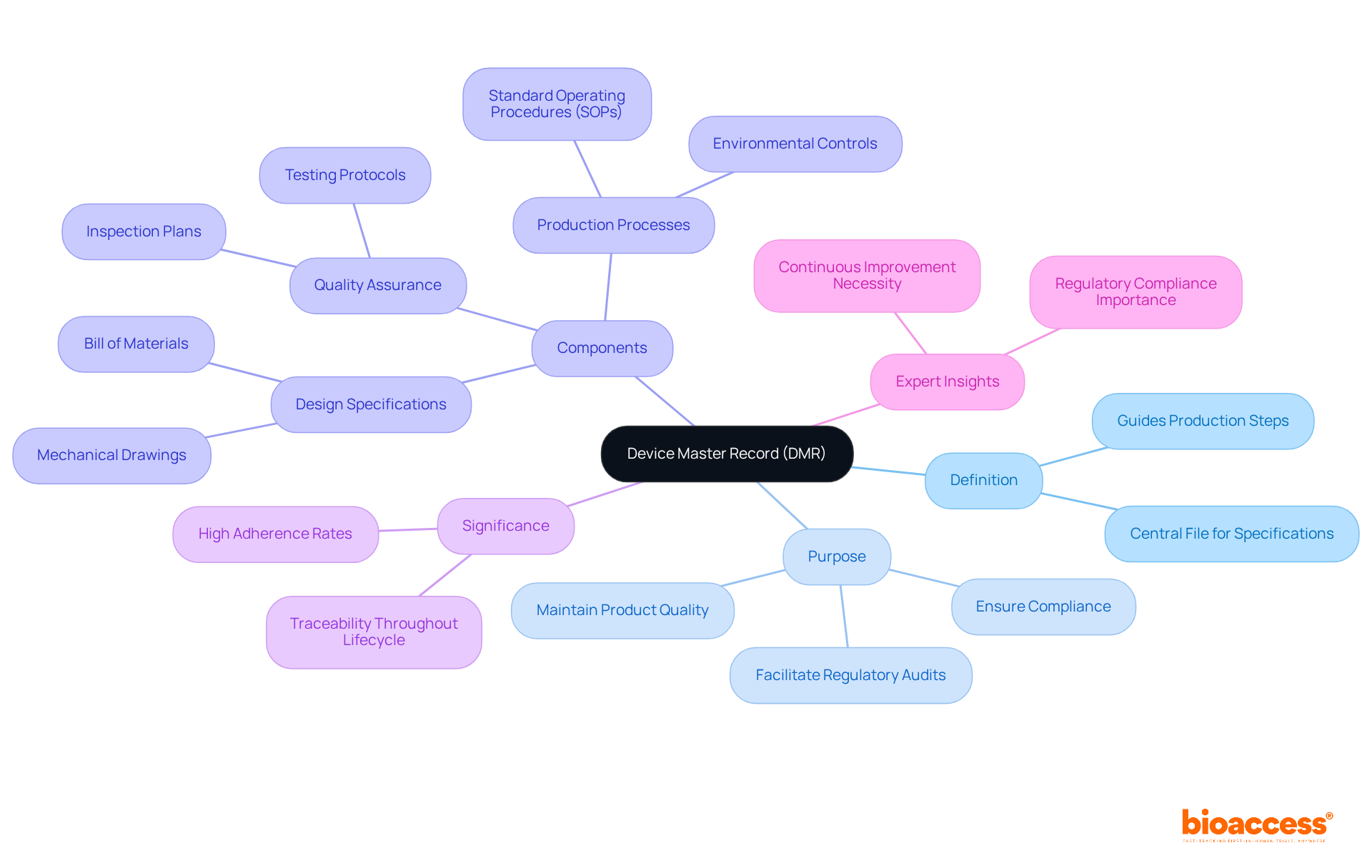
A Device Master Record (DMR) is an essential collection of documents and specifications required for the production of a medical product. It serves as the official 'recipe' for production, encompassing everything from design specifications to quality assurance protocols. The primary purpose of a DMR is to ensure that every aspect of the manufacturing process is meticulously documented and standardized. This is vital for compliance with regulatory requirements and for maintaining product quality. This centralized record is crucial for ensuring consistency and traceability throughout the product's lifecycle.
Effective documentation in biopharma exemplifies this principle by integrating comprehensive records that encompass specifications, production processes, and quality assurance measures. A well-structured DMR typically includes controlled documents organized by product family, ensuring that all necessary elements are readily accessible and compliant with regulations such as 21 CFR 820.181.
The significance of device master records cannot be overstated; they are essential for achieving high adherence rates in the medical equipment sector. Current data indicates that manufacturers with robust device master records experience markedly higher adherence rates during audits compared to those that do not have device master records. This underscores the importance of maintaining device master records to facilitate seamless regulatory audits and ensure ongoing compliance.
Regulatory experts, including Ana Criado, Director of Regulatory Affairs and a consultant with extensive experience in biomedical engineering and health economics, emphasize that device master records are the backbone of manufacturing operations. It guides every production step, ensuring that each device produced aligns with the original design specifications. As noted by ISO 13485, 'A robust DMR is indispensable for demonstrating adherence to FDA regulations.' Thus, the DMR not only supports operational excellence but also plays a pivotal role in safeguarding patient safety and product efficacy. Continuous enhancement and ongoing oversight of the DMR are essential for upholding standards and adapting to evolving regulatory landscapes.
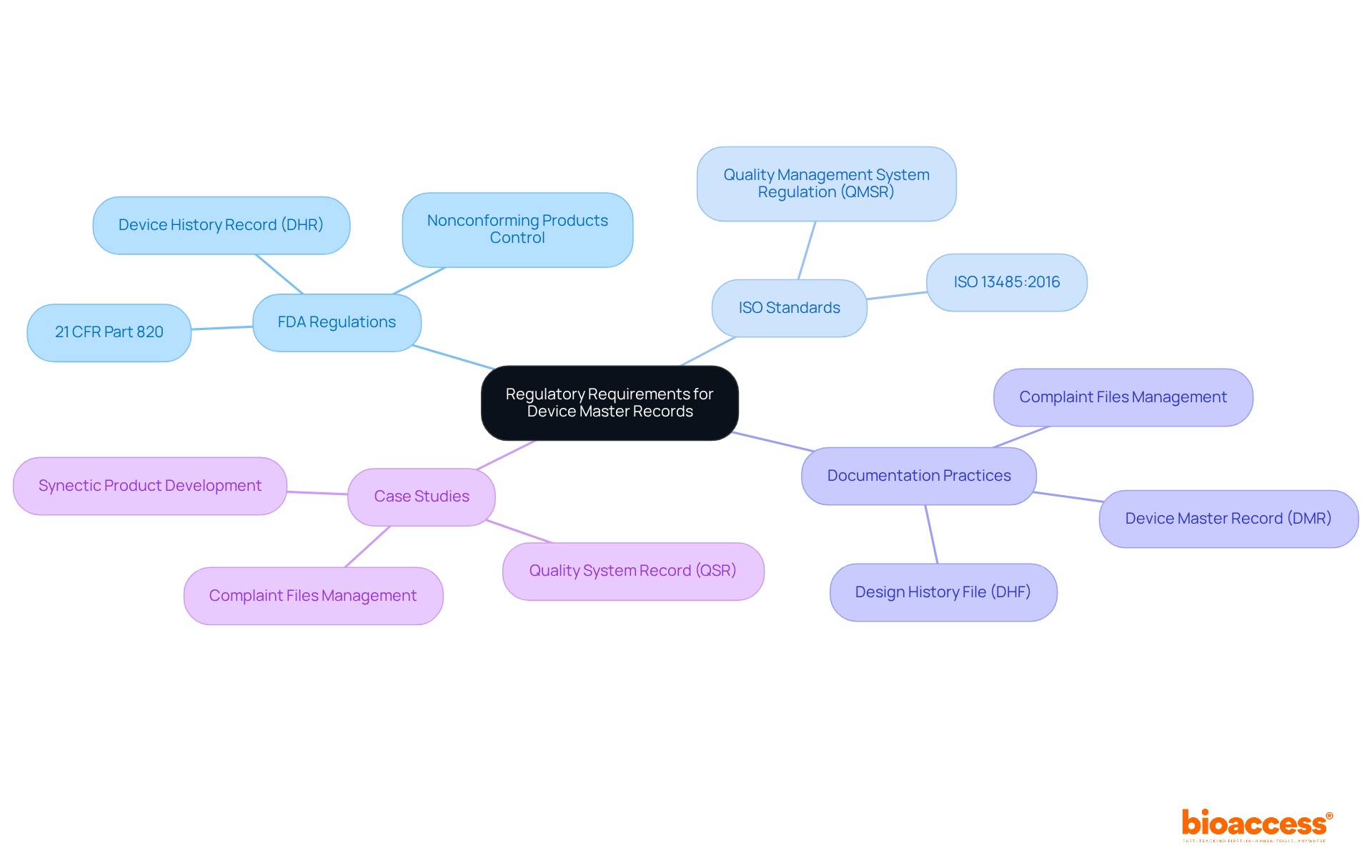
Device master records are subject to rigorous regulatory requirements established by the FDA and ISO standards. The FDA mandates adherence to 21 CFR Part 820, which outlines the quality system regulations for medical equipment. This regulation necessitates that manufacturers maintain precise and comprehensive records documenting the device's design, production, and quality assurance processes. Furthermore, manufacturers are required to establish procedures for controlling nonconforming products, ensuring both adherence and product safety.
ISO 13485:2016 highlights the critical role of documentation in achieving consistent quality and regulatory compliance. Manufacturers must ensure that their device master records (DMRs) are not only accurate but also regularly updated and reviewed to align with these evolving standards. Notably, the FDA's recent synchronization of its Quality Management System Regulation (QMSR) with ISO 13485, effective February 2, 2026, aims to simplify adherence across markets, emphasizing the necessity for manufacturers to adjust their documentation practices accordingly.
As noted by Luana Zerafa, Manager of Quality & Regulatory Affairs Program, maintaining a robust device master records (DMR) is essential for demonstrating compliance and ensuring product safety throughout the product lifecycle. Case studies from medical equipment firms illustrate that effective management of device master records can significantly enhance operational efficiency and regulatory adherence, ultimately leading to successful product commercialization. Additionally, manufacturers must document acceptance activities as part of the Device History Record (DHR), with records retained for a minimum of two years from the date of commercial distribution.
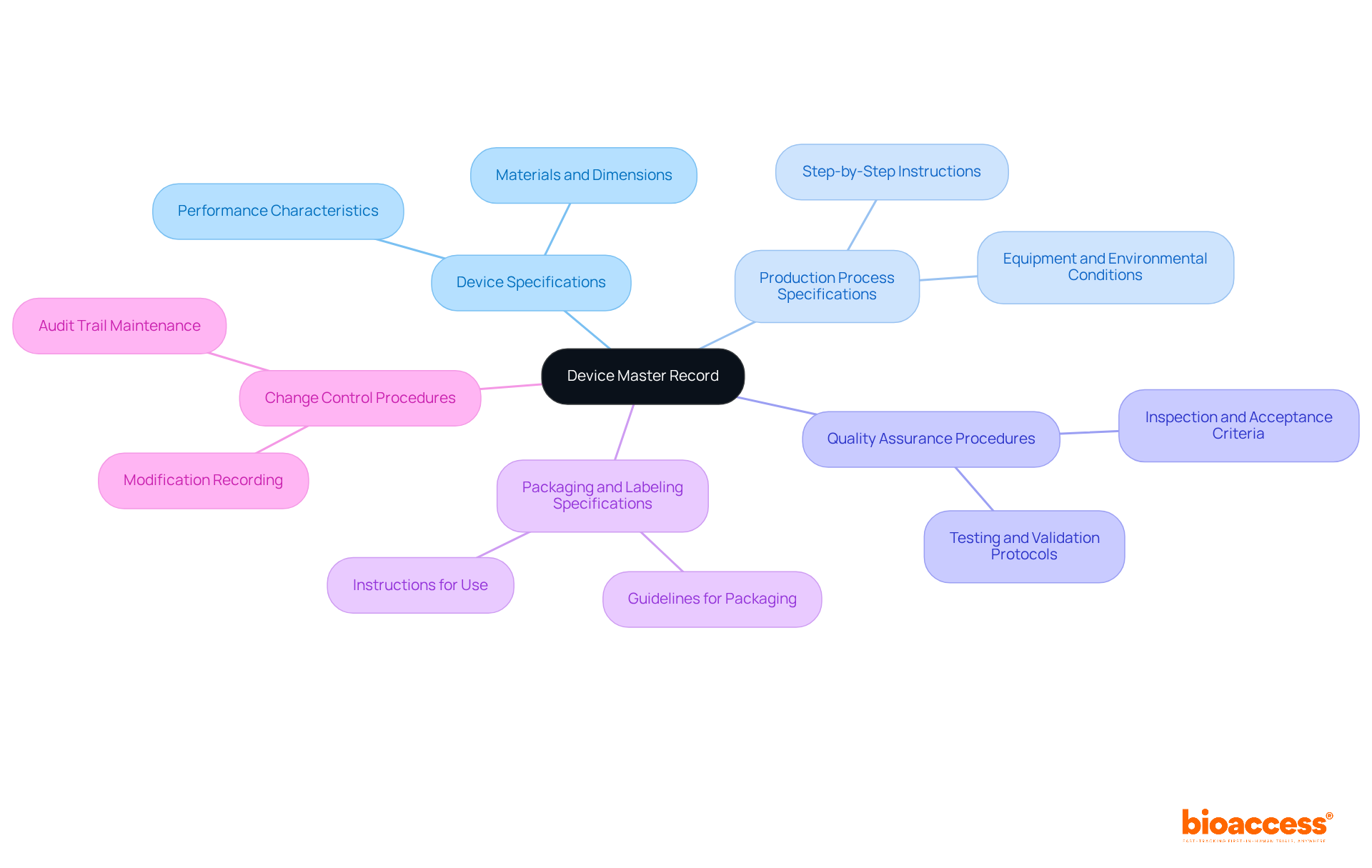
A comprehensive device master records is essential for ensuring regulatory compliance and effective manufacturing processes. It must encompass several critical components:
Device Specifications: This section provides detailed descriptions of the device, including materials, dimensions, and performance characteristics. Accurate specifications are crucial for maintaining consistency and quality throughout the manufacturing process.
Production Process Specifications: Step-by-step instructions for creating the apparatus must be included, detailing the equipment used and the environmental conditions required. This ensures that all production activities are standardized and traceable.
Quality Assurance Procedures: Protocols for testing and validating the apparatus are vital to confirm that it meets safety and efficacy standards. These procedures should outline inspection criteria and acceptance criteria to ensure compliance with quality regulations.
Packaging and Labeling Specifications: Guidelines for packaging and labeling the product must be clearly defined, including instructions for use. Proper packaging ensures the device's safety and sterility during transportation and storage.
Change Control Procedures: Recording any modifications to the DMR is vital for ensuring traceability and adherence to regulatory requirements. This includes maintaining an audit trail of modifications to support ongoing quality assurance efforts.
In 2025, device master records must reflect the latest industry standards and regulatory expectations, incorporating comprehensive documentation that supports both pre-market and post-market activities. Quality assurance experts emphasize that a well-organized DMR not only aids in compliance but also enhances the overall quality and safety of medical equipment.
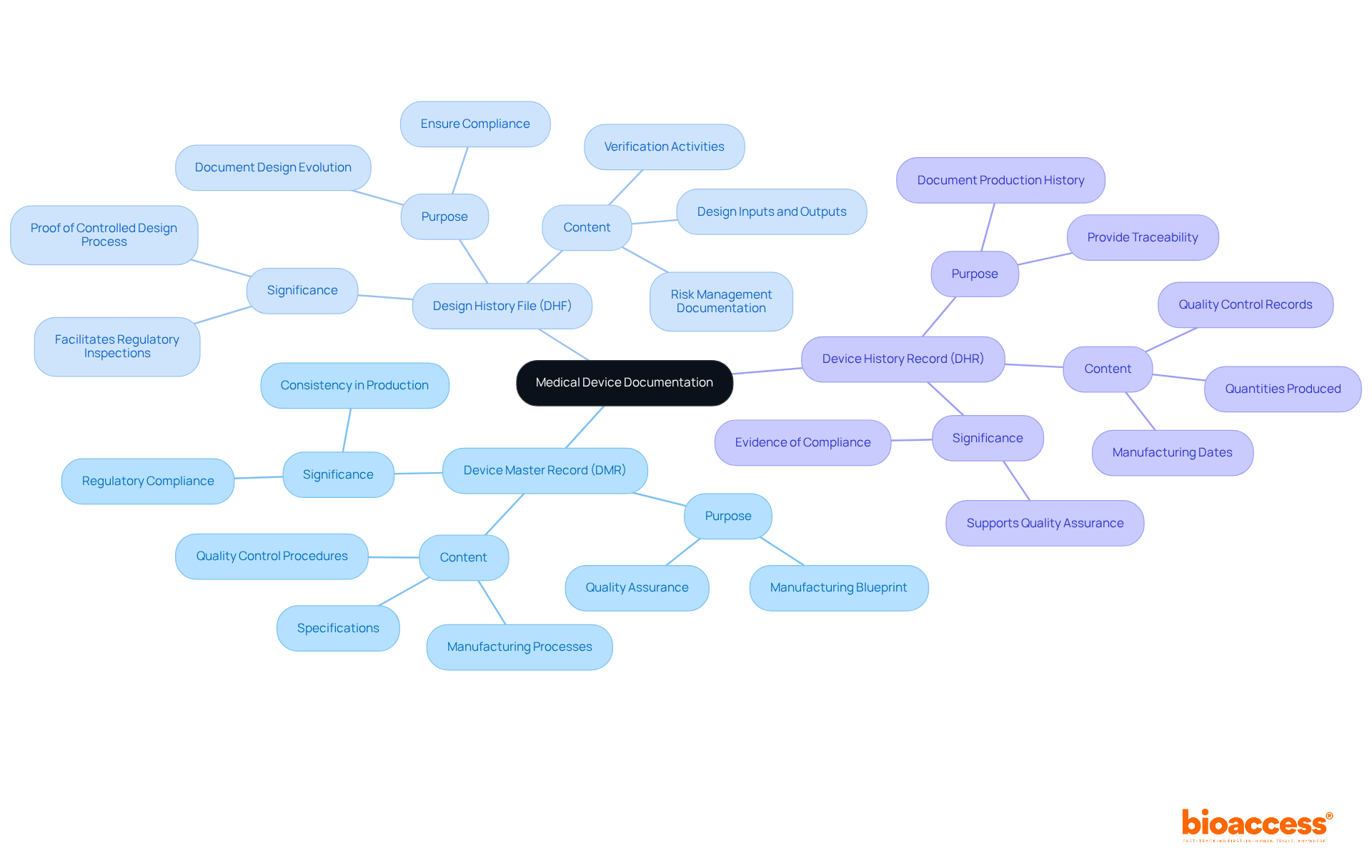
The device master records, along with the Design History File (DHF) and Device History Record (DHR), are critical components of medical device documentation, each serving a unique purpose.
Device master records: This document outlines the specifications and procedures necessary for manufacturing the device. It serves as the formal 'recipe' for production, detailing everything from equipment specifications to quality assurance processes, thereby ensuring consistent manufacturing practices.
Design History File (DHF): The DHF encompasses all documentation related to the design and development of the product. It includes design inputs, outputs, verification activities, and risk management documentation, providing a thorough account of the product's evolution from concept to final design.
Device History Record (DHR): The DHR captures the production history of a specific item, documenting manufacturing dates, quantities produced, and quality control records. This record serves as evidence that each unit was produced in accordance with the specifications outlined in the device master records.
Understanding the distinct roles of the device master records, design history file, and device history record is crucial for maintaining compliance with regulatory standards and ensuring the integrity of the medical product lifecycle. Proper documentation practices not only facilitate regulatory inspections but also enhance product quality and safety, as emphasized by industry experts. Furthermore, with the impending transition to the Quality Management System Regulation (QMSR) and the introduction of the Medical Device File (MDF), it is essential for manufacturers to adapt their documentation strategies accordingly. Statistics indicate that organizations adopting robust documentation practices experience a significant reduction in compliance-related issues, underscoring the importance of these records in the medical equipment sector.
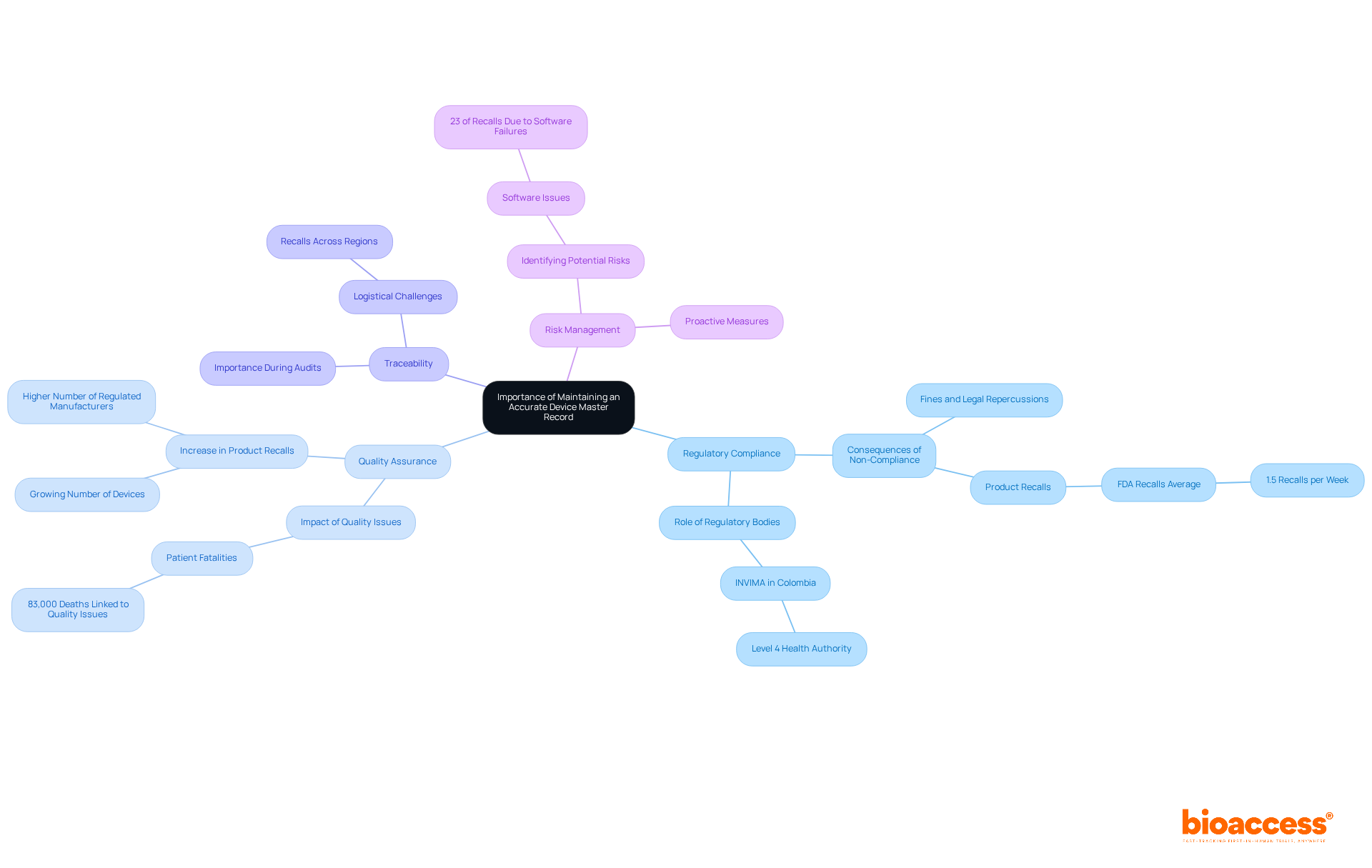
Maintaining an accurate Device Master Record (DMR) is essential for several reasons:
Regulatory Compliance: Inaccurate or incomplete DMRs can lead to significant regulatory non-compliance, resulting in fines, product recalls, and even legal repercussions. For instance, the FDA announces an average of 1.5 product recalls each week, many arising from inadequate documentation practices. In Colombia, INVIMA plays a crucial role in overseeing these regulations, ensuring that health products meet safety and efficacy standards as a Level 4 health authority recognized by PAHO/WHO.
Quality Assurance: A precise device master records (DMR) ensures that all manufacturing processes adhere to established specifications, thereby reducing the risk of defects. Over the past decade, medical equipment quality issues have been linked to 83,000 patient fatalities, underscoring the essential requirement for rigorous quality controls. The increase in product recalls is also attributed to a growing number of devices on the market and a higher number of regulated manufacturers, including those under INVIMA's jurisdiction.
Traceability: A well-maintained device master records (DMR) system facilitates easy tracking of changes and updates, which is crucial during audits and inspections. This traceability is vital for identifying the root causes of any issues that may arise. Logistical difficulties may occur for recalls distributed across various regions or nations, making precise documentation even more essential. Specialists such as Ana Criado, who possesses significant expertise in regulatory matters at INVIMA, emphasize the importance of maintaining detailed records for adherence and traceability.
Risk Management: Accurate records are instrumental in identifying potential risks within the manufacturing process, allowing for proactive measures to mitigate them. For example, software issues have been identified as the leading cause of product recalls, accounting for 23% of recalls in a recent quarter. Moreover, software problems are the primary cause of recalled items, highlighting the importance of precise device master records in managing risks.
Overall, the integrity of device master records (DMR) directly influences the safety and efficacy of medical devices, establishing them as a cornerstone of effective clinical research and regulatory adherence. Incorporating perspectives from regulatory officers about the dangers of erroneous data management reports would further strengthen the credibility of this discussion.
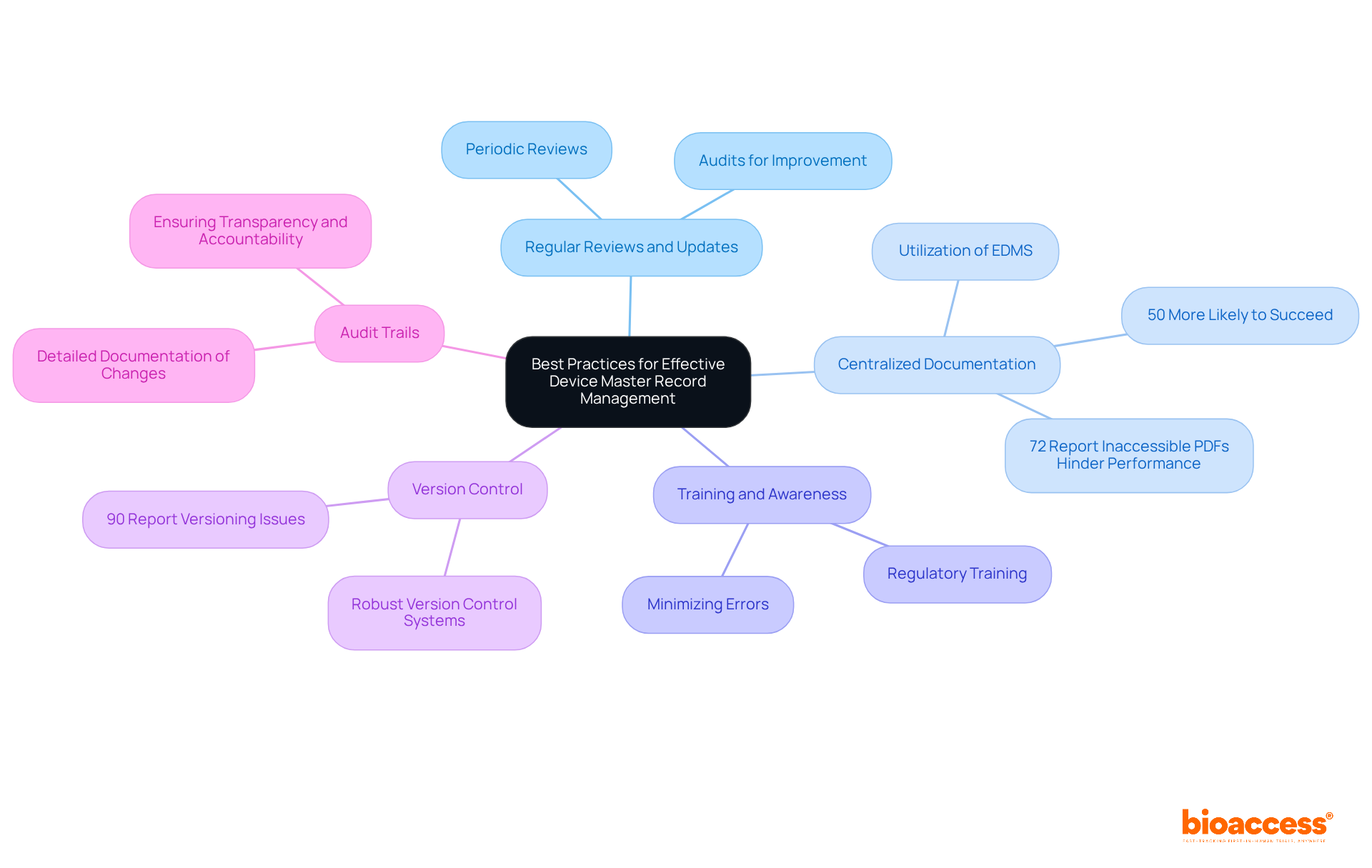
To effectively manage device master records, manufacturers must adopt best practices that ensure compliance and operational excellence in the highly regulated medical device industry.
Regular Reviews and Updates: Schedule periodic reviews of the DMR to ensure all information remains current and accurate. Regular audits assist in recognizing gaps and areas requiring enhancement, thereby improving adherence and operational efficiency. Frequent evaluations of data management records not only enhance compliance with regulations but also demonstrate a commitment to maintaining high standards in data management.
Centralized Documentation: Utilize a centralized electronic document management system (EDMS) to store and manage device master records. This approach facilitates easy access and collaboration, ensuring that all team members are working with the most up-to-date information. Companies utilizing centralized product repositories are over 50% more likely to succeed in upholding regulations. Additionally, 72% of employees report that inaccessible PDFs hinder their job performance, underscoring the critical importance of effective document management systems.
Training and Awareness: Ensure that all team members involved in DMR management are well-trained on regulatory requirements and best practices. This training is essential for maintaining compliance and minimizing the risk of errors due to insufficient understanding.
Version Control: Implement a robust version control system to track changes and maintain a clear history of document revisions. With 90% of businesses reporting document versioning issues, effective version control tools can significantly reduce time wasted on outdated documents.
Audit Trails: Maintain detailed audit trails to document all changes made to the device master records. This practice guarantees transparency and accountability, which are vital for adherence to FDA and international regulations.
By integrating these strategies, manufacturers can significantly enhance their DMR management processes, ensuring compliance and operational excellence. As noted by the Aberdeen Group, "best-in-class companies significantly outperform laggards in new product development," highlighting the critical need for adopting these best practices.
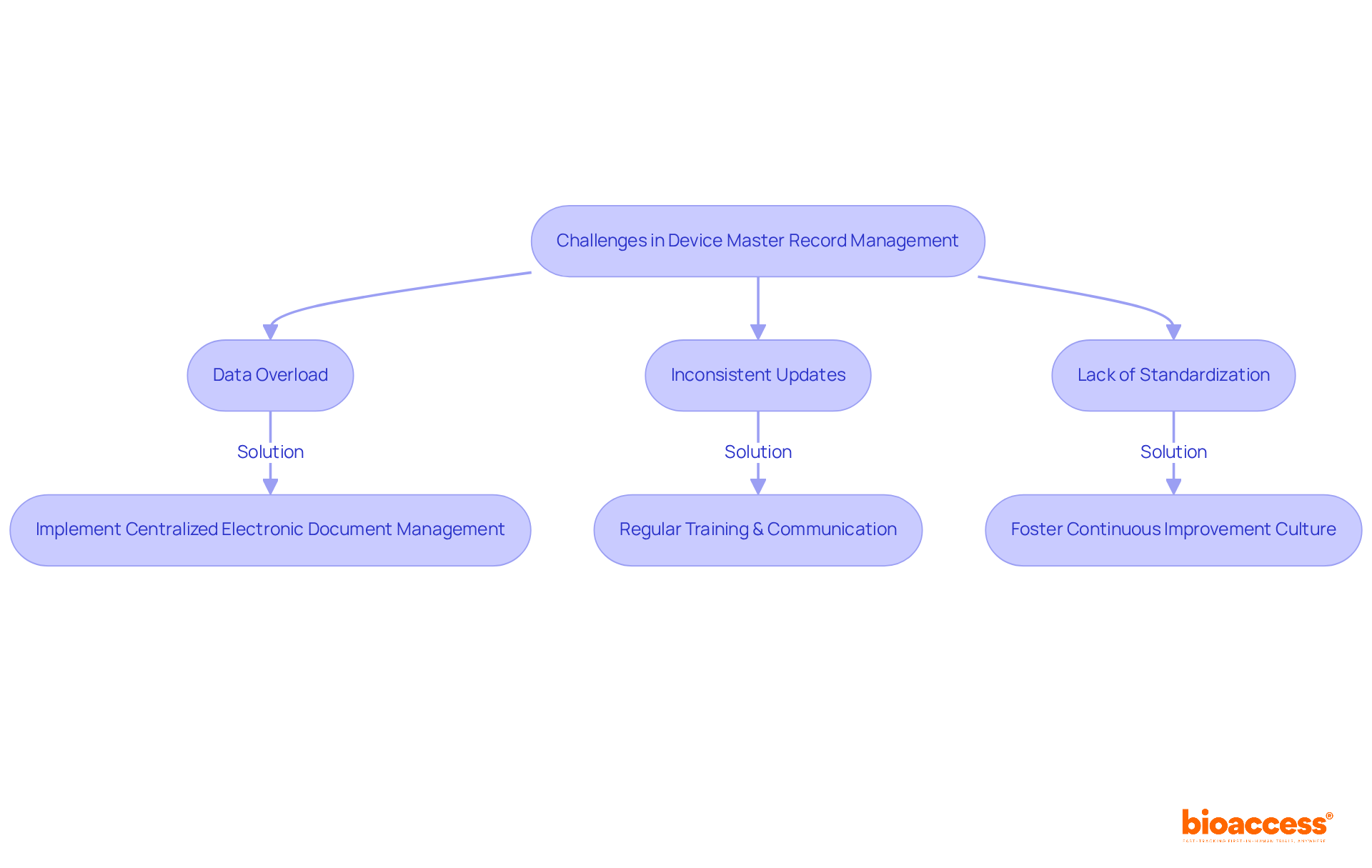
Managing device master records presents several significant challenges that can impact compliance and operational efficiency.
Data overload is a critical issue; the extensive volume of documentation often leads to confusion and errors, complicating the maintenance of accurate records. Many entities struggle to keep data management reports precise and readily available, which obstructs regulatory initiatives. Indeed, most manufacturers still manage Device History Records (DHRs) manually, relying on paper documentation, exacerbating this issue.
Inconsistent updates also pose a risk. Regularly assessing and enhancing DMR processes is crucial. Failing to refresh the device master records may result in outdated information, jeopardizing adherence to regulations and potentially leading to costly legal problems. Allison Dunn emphasizes that jumping to conclusions without understanding the root cause can lead to significant problems in DMR management.
Moreover, the lack of standardization complicates matters. Without standardized processes, data management reports can vary greatly between projects, complicating audits and heightening the risk of non-compliance. This inconsistency creates challenges in maintaining quality assurance across different teams and projects.
To address these challenges, implementing a centralized electronic document management system can streamline device master records management, ensuring consistency and accuracy across all records. Such systems enhance traceability by linking DMRs to Device History Records (DHRs), enabling verification for each lot or batch. Furthermore, regular training and clear communication among team members are essential to mitigate these challenges. By fostering a culture of adherence and continuous improvement, organizations can navigate the complexities of DMR management more effectively and enhance their operational efficiency. A case study on 'Key Takeaways for Device Master Record Success' illustrates how effective DMR implementation can lead to improved adherence and operational outcomes.
Utilizing technology in managing device master records results in significant enhancements in efficiency and adherence. Key technological solutions include:
Electronic Document Management Systems (EDMS): These systems streamline the creation, storage, and retrieval of DMRs, ensuring that all documents are easily accessible and consistently up-to-date. Research indicates that implementing EDMS can reduce document processing time by up to 55%, significantly enhancing operational efficiency. Additionally, investments in document management automation yield a 248% return within three years, underscoring the financial benefits of adopting device master records for these solutions.
By incorporating automated workflows, organizations can accelerate the review and approval processes for device master records updates, thereby reducing timelines for adherence. This automation not only accelerates operations but also enhances accuracy, with studies showing an 88% improvement in document accuracy through effective integration. As noted by Forrester, "implementing electronic document management reduces document processing time by 55% and boosts employee productivity by 45%."
Utilizing data analytics tools enables organizations to identify trends and potential issues in device master records management proactively. This capability supports informed decision-making by utilizing device master records, allowing teams to address challenges before they escalate.
Cloud-based solutions streamline the management of device master records, enabling real-time collaboration among team members, regardless of their location. This enhances communication and efficiency in the management of device master records, as 72% of businesses recognize remote file sharing and access as a critical benefit of digital document processes. Furthermore, 44% of businesses identified remote file sharing as the most important benefit of digital document processes. The adaptability of cloud solutions also facilitates adherence to regulatory requirements, making it easier to manage device master records securely.
Incorporating these technologies not only streamlines device master records management but also effectively positions organizations to meet the evolving demands of the medical technology landscape. A practical takeaway for organizations is to assess their current document management processes and consider implementing these technological solutions to enhance efficiency and compliance.

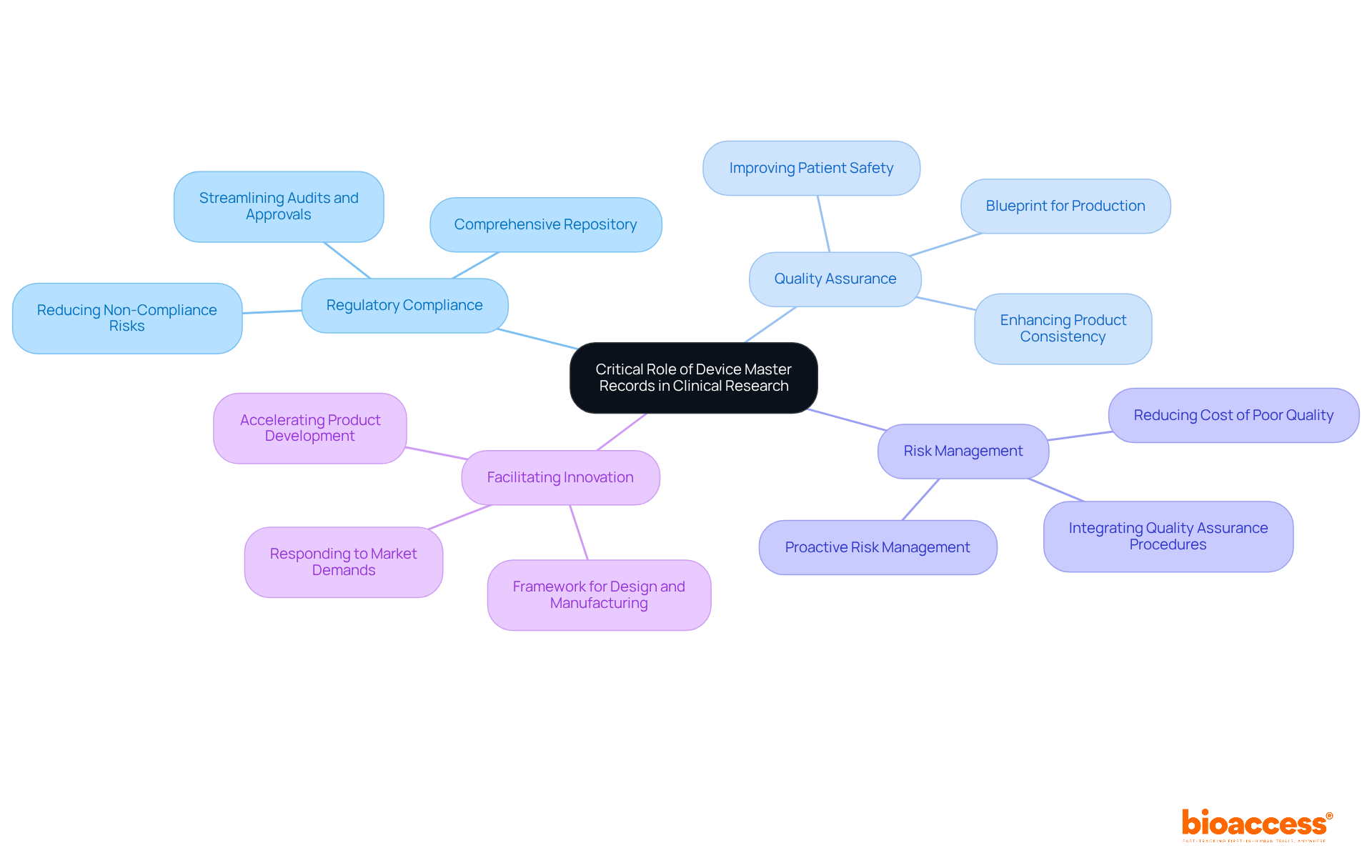
Device master records are essential in clinical research, serving as the foundation for documenting and standardizing every aspect of a medical device's development. Their significance is underscored by several key factors:
Regulatory Compliance: DMRs are crucial for demonstrating adherence to regulatory standards, streamlining audits and approvals. They serve as a comprehensive repository of device master records, which is essential for meeting the stringent expectations set by regulatory bodies like the FDA. A well-maintained device master records can significantly reduce the risk of non-compliance, as evidenced by the fact that organizations with robust quality assurance programs often experience recall rates below 0.2% of shipped units.
Quality Assurance: By meticulously detailing manufacturing processes and specifications, device master records are crucial in maintaining product quality and safety. They serve as a blueprint for product production, ensuring that every aspect of the manufacturing process is documented and controlled. This level of detail not only enhances product consistency but also fosters collaboration across teams, ultimately leading to improved patient safety.
Risk Management: Precise and thorough DMRs enable manufacturers to recognize and reduce risks linked to product production. They facilitate proactive risk management by integrating quality assurance procedures and acceptability criteria, which are essential for compliance with quality standards and the management of device master records. This approach can lead to a significant reduction in the Cost of Poor Quality (CoPQ), often halving total costs associated with defects.
Facilitating Innovation: A well-maintained DMR accelerates the development and iteration of medical products, enabling quicker responses to market demands and technological advancements. By offering a clear framework for design and manufacturing, these guidelines foster innovation while ensuring that safety and efficacy standards are met. This agility is crucial in a rapidly evolving industry where timely market entry can be a competitive advantage.
In summary, device master records are not merely regulatory requirements; they are essential tools that enhance quality assurance, foster innovation, and guarantee compliance in the medical equipment industry. Additionally, with bioaccess's expertise in managing clinical trials across various stages—including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies—device master records play a critical role in post-market surveillance, allowing manufacturers to track device performance and make necessary enhancements, further solidifying their importance throughout the device lifecycle.
Device master records (DMRs) are pivotal in clinical research, serving as the backbone for ensuring compliance, quality assurance, and innovation in medical device development. Their integral role in streamlining regulatory processes and enhancing operational efficiency is paramount. By centralizing and meticulously documenting every aspect of a device's lifecycle, DMRs facilitate faster approvals and patient recruitment while safeguarding the integrity and safety of medical products.
Key insights from the article underscore the necessity of maintaining accurate and comprehensive device master records. This encompasses:
Furthermore, the article emphasizes the importance of leveraging technology and best practices in managing DMRs to mitigate common challenges and enhance compliance.
Ultimately, the significance of device master records extends beyond mere regulatory compliance; they are essential tools that foster innovation, enhance patient safety, and improve clinical research outcomes. Organizations involved in medical device manufacturing and clinical trials must prioritize the development and maintenance of robust DMR systems to navigate the complexities of the industry effectively. By doing so, they not only uphold regulatory standards but also contribute to the advancement of healthcare and the successful introduction of new medical technologies to the market.
What is bioaccess® and how does it relate to clinical research?
bioaccess® is an organization that integrates device master records to optimize clinical research workflows, accelerating both the approval and enrollment phases of clinical trials by centralizing essential documentation.
What is a Device Master Record (DMR)?
A Device Master Record (DMR) is a collection of documents and specifications required for the production of a medical product, serving as the official 'recipe' for production, including design specifications and quality assurance protocols.
Why are Device Master Records important in clinical research?
Device Master Records are crucial for ensuring compliance with regulatory requirements, maintaining product quality, and safeguarding the integrity of clinical research, ultimately enhancing patient outcomes and expediting market access for innovative medical products.
What regulatory requirements govern Device Master Records?
Device Master Records are subject to regulatory requirements established by the FDA, specifically 21 CFR Part 820, and ISO 13485:2016 standards, which mandate precise documentation of design, production, and quality assurance processes.
How do Device Master Records contribute to operational efficiency?
Effective management of Device Master Records minimizes manufacturing mistakes, enhances operational efficiency, and upholds regulatory compliance, thereby reinforcing the commitment to producing safe and effective medical products.
What role do Device Master Records play in regulatory audits?
Manufacturers with robust Device Master Records experience higher adherence rates during audits, facilitating seamless regulatory reviews and ensuring ongoing compliance with established standards.
How often should Device Master Records be updated?
Device Master Records should be regularly updated and reviewed to align with evolving regulatory standards and ensure accuracy in documentation practices.
What is the significance of the FDA's synchronization of its Quality Management System Regulation with ISO 13485?
This synchronization, effective February 2, 2026, aims to simplify adherence across markets, emphasizing the necessity for manufacturers to adjust their documentation practices accordingly.
What must manufacturers document as part of the Device History Record (DHR)?
Manufacturers must document acceptance activities as part of the Device History Record, retaining records for a minimum of two years from the date of commercial distribution.