


The article emphasizes the critical benefits of effective device listing for clinical success, asserting that accurate and validated device listings are essential for regulatory compliance, market access, and patient safety. It elaborates on how precise listings bolster credibility, streamline reimbursement processes, facilitate market intelligence, and stimulate innovation. Ultimately, these factors contribute to enhanced outcomes for medical technologies and improved patient care. By understanding the significance of these elements, stakeholders can better navigate the complexities of the Medtech landscape, ensuring that they meet the challenges of clinical research head-on.
Effective device listings transcend mere regulatory requirements; they are essential to the success of medical technologies within a rapidly evolving healthcare landscape. By ensuring regulatory compliance, fostering trust, and enabling market access, these listings act as a cornerstone for Medtech, Biopharma, and Radiopharma companies striving to excel. As the stakes escalate with increasing scrutiny and potential penalties, organizations must consider how to leverage these listings not only to achieve compliance but also to gain a competitive edge and enhance patient safety. This article explores the seven key benefits of effective device listings, illuminating their critical role in achieving clinical success.
bioaccess® leverages its comprehensive understanding of regulatory frameworks across Latin America, the Balkans, and Australia to expedite swift medical product approvals. This capability not only guarantees that product entries are precise and compliant, but also significantly shortens the duration required to introduce innovative medical technologies. Remarkably, ethical approvals are frequently obtained in just 4-6 weeks. Such efficiency is vital for Medtech, Biopharma, and Radiopharma companies that aim to capitalize on emerging opportunities within the healthcare sector. By streamlining the approval process, bioaccess® positions itself as an essential partner in navigating the complexities of clinical research.

Regulatory compliance is paramount for the successful promotion of medical equipment. Precise device listings are essential, as they provide regulatory authorities with all necessary information for market entry. Firms that maintain current and accurate records can significantly mitigate risks associated with non-compliance, including penalties and product recalls.
In 2025, the ramifications of non-compliance are anticipated to be more pronounced, with heightened scrutiny resulting in an increased likelihood of recalls. Authorities in clinical research management recommend conducting regular evaluations of equipment records to ensure adherence and facilitate a more seamless approval process.
This proactive approach not only enhances market access but also fortifies the integrity of the product's compliance status, ultimately contributing to clinical success.
Validated device listings serve as a testament to the quality and safety of medical apparatus. When devices are part of a device listing with trustworthy authorities such as INVIMA, the Colombia National Food and Drug Surveillance Institute, their reputation among healthcare providers, patients, and investors is significantly enhanced.
INVIMA's crucial role in inspecting and supervising health products, along with its classification as a Level 4 health authority by PAHO/WHO, underscores the necessity of regulatory compliance in fostering trust. This trust is particularly essential in an environment where safety and efficacy are paramount.
The Clinical Research Management Specialist emphasizes that companies must prioritize securing validation for their product registrations to establish trust and facilitate easier entry into the industry.
For instance, Welwaze Medical Inc. has engaged bioaccess™ as its regulatory and market access consulting firm to aid in the introduction of the Celbrea® medical product in Colombia, highlighting the critical role of the device listing in achieving successful market integration.
Efficient product catalogs significantly enhance a firm's competitive advantage. By providing comprehensive and transparent information regarding a product's features, benefits, and compliance status, companies can attract greater interest from healthcare professionals and decision-makers. The Clinical Research Management Specialist emphasizes that a strategic approach to product cataloging—encompassing SEO optimization and targeted promotion—can enable companies to capture a larger share of the market.
Device listing catalogs are vital for ensuring patient safety, as they provide essential information regarding a device's intended use, contraindications, and potential risks. Accurate device listings empower healthcare providers to make informed decisions, ultimately safeguarding patients from harm.
In Latin America, navigating the legal landscape, particularly with agencies like INVIMA in Colombia, is crucial. Recognized as a Level 4 health authority by PAHO/WHO, INVIMA plays a significant role in overseeing medical instruments to ensure compliance with safety and efficacy standards.
The Clinical Research Management Expert emphasizes that maintaining stringent standards for device registrations transcends mere regulatory obligation; it is a moral imperative to protect patient health. By understanding and adhering to these regulations, organizations like bioaccess® can facilitate smoother market entry and enhance the safety of medical technologies in the region.
Device listings serve as a critical source of industry intelligence, providing insights into competitor products, pricing strategies, and emerging trends. By analyzing the data contained within product descriptions, organizations can identify market gaps and opportunities for innovation. The Clinical Research Management Expert emphasizes that utilizing this data can inform strategic decisions and bolster a company's competitive positioning.
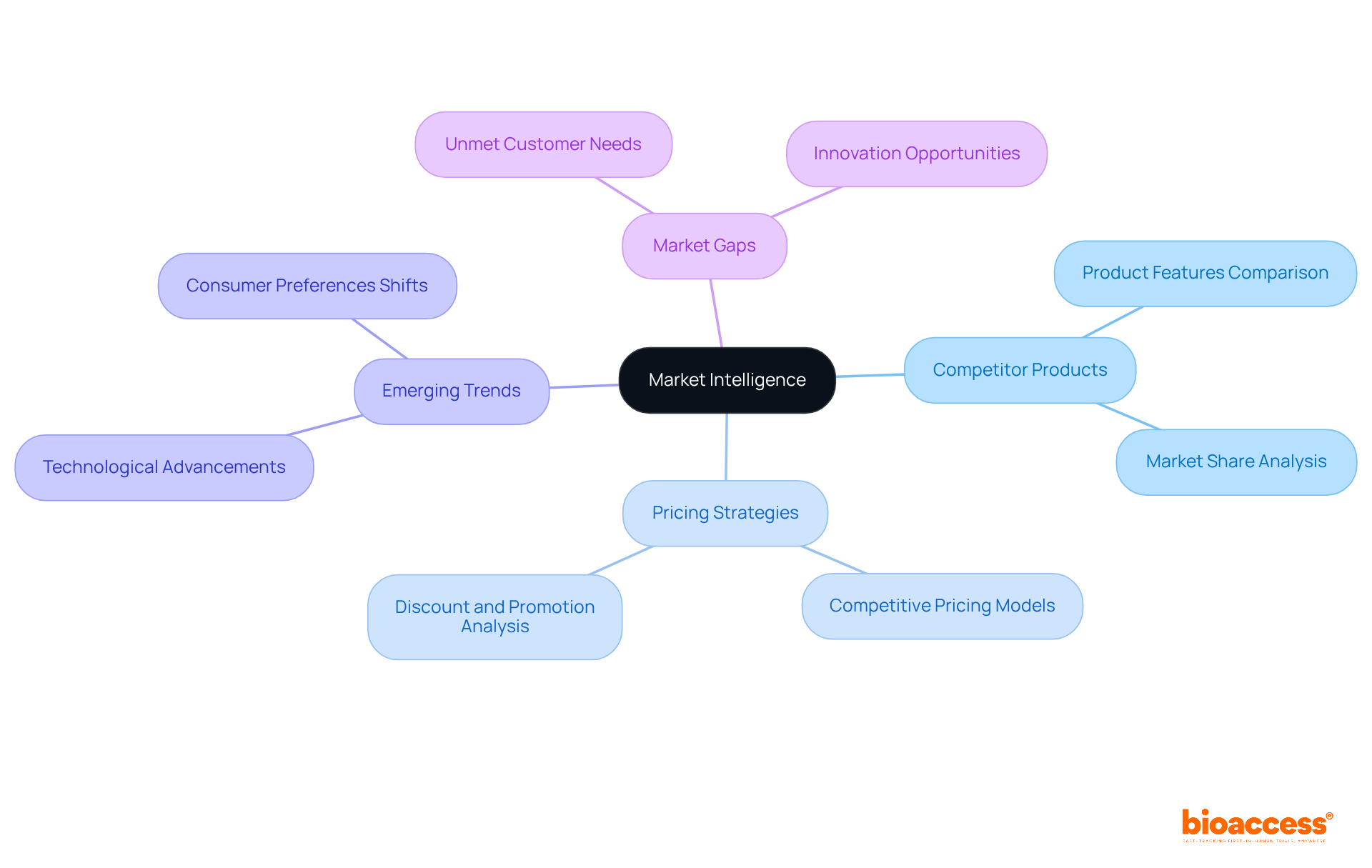
Precise equipment records are essential for optimizing reimbursement procedures within the medical equipment sector. By delivering comprehensive and clear information, companies can significantly expedite claims, minimizing payment delays. Financial experts note that efficient product presentations enhance interactions with payers, which is critical for facilitating smoother financial exchanges. This clarity not only accelerates the reimbursement cycle but also enhances patient access to vital medical technologies.
As the healthcare landscape evolves, the importance of accurate device listings becomes increasingly evident, underscoring their role in promoting prompt financial procedures and supporting the overall success of clinical initiatives.
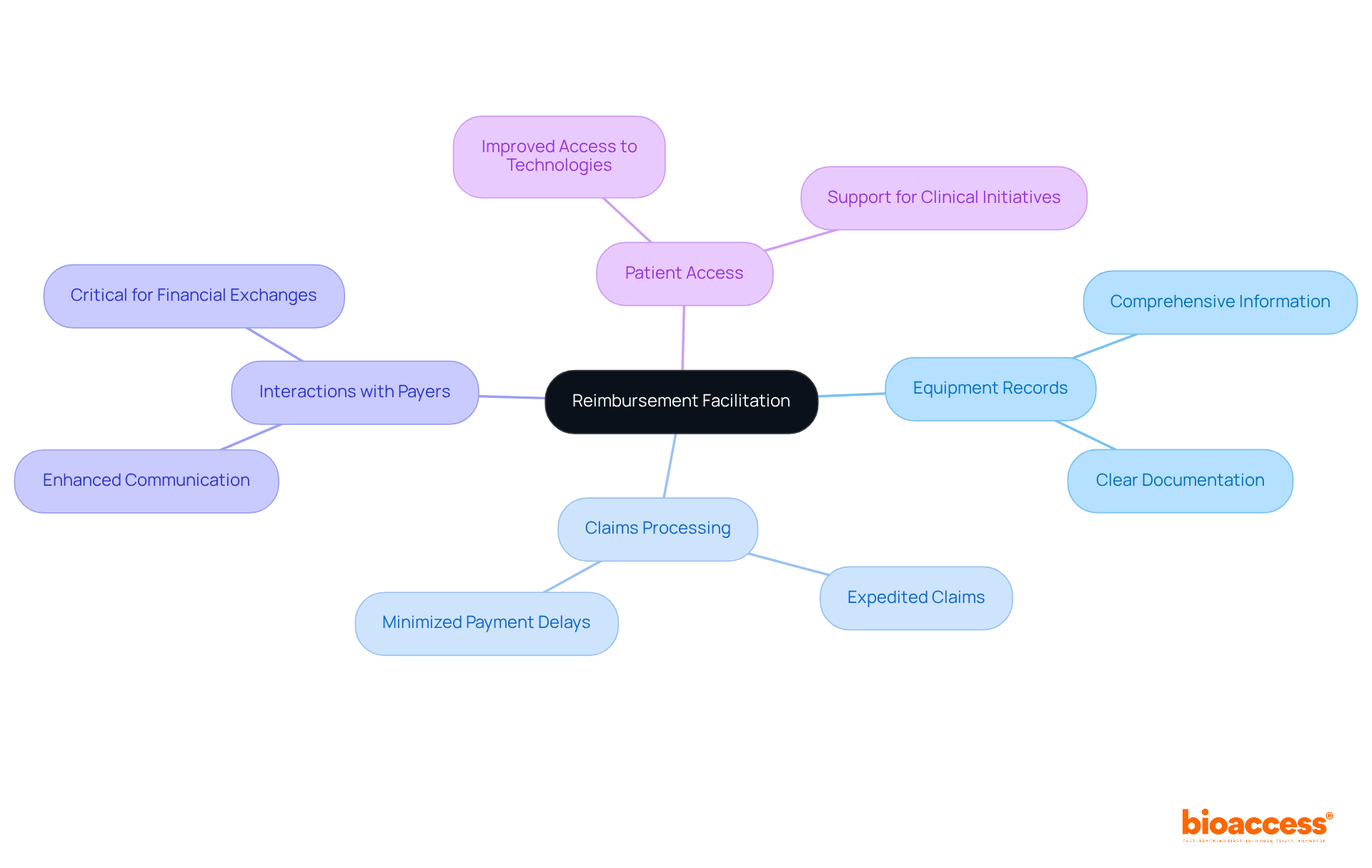
To guarantee adherence to regulatory standards, it is essential for firms to maintain an updated device listing. Regulatory changes, product modifications, and new safety data must be accurately represented in the device listing to avoid penalties and maintain market access. Specialists advocate for the establishment of a structured evaluation procedure to regularly refresh equipment records, significantly minimizing compliance risks.
For instance, firms like Intuitive Surgical have adeptly managed modifications in their product catalogs by implementing proactive strategies that align with compliance updates. As Bob Mehta, a primary consultant in regulatory compliance, emphasizes, 'A structured method for examining product entries is crucial for maneuvering through the intricacies of regulatory obligations.'
Furthermore, organizations must acknowledge the annual registration requirement from October 1 to December 31, underscoring the critical nature of maintaining equipment records. Failing to uphold current records can lead to substantial compliance risks, including penalties or removal by the FDA. Staying informed about contemporary trends in compliance updates is vital for organizations aiming to enhance their clinical success while ensuring the safety and effectiveness of their medical products.
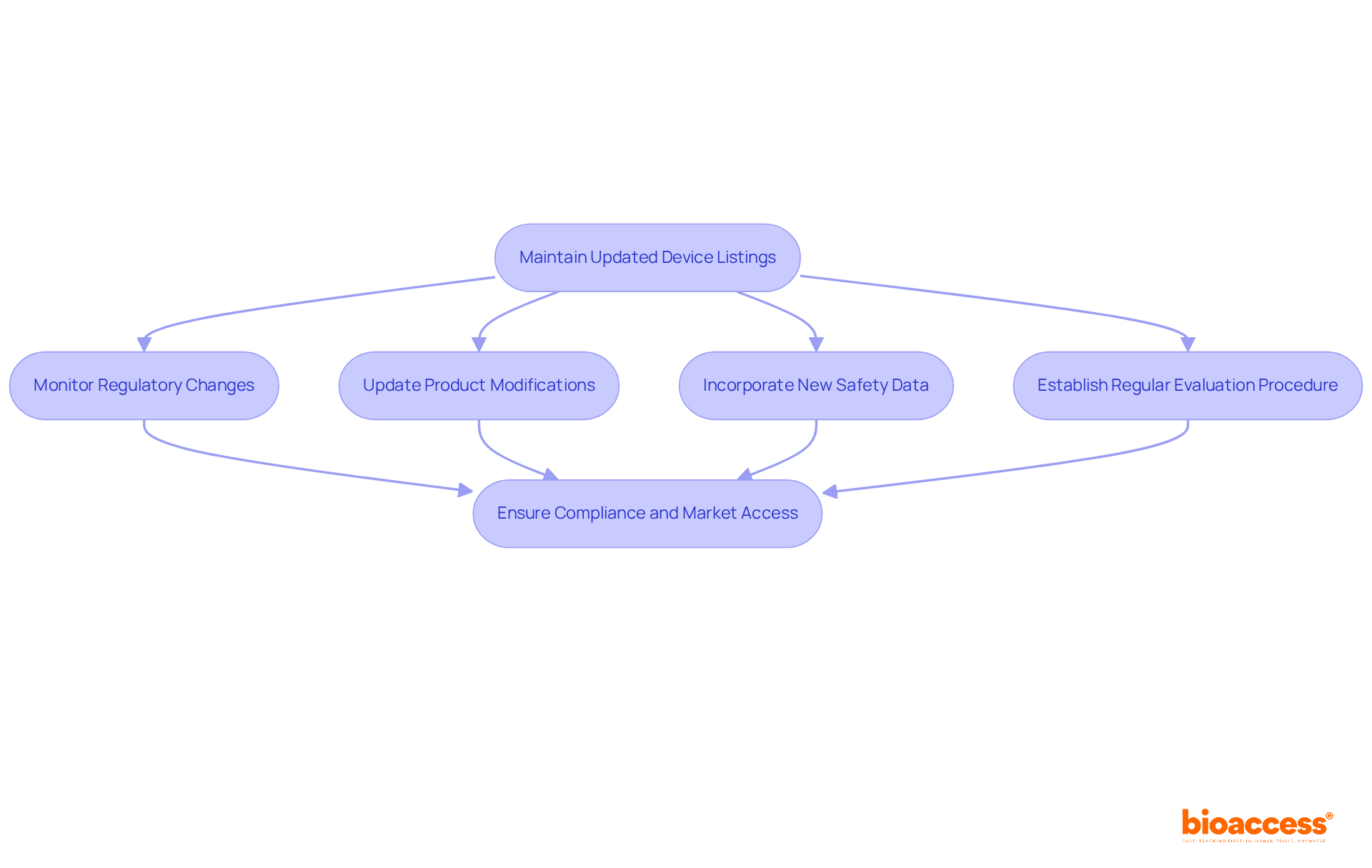
Effective device descriptions foster an innovation-friendly environment by establishing clear guidelines and expectations for new medical technologies. Understanding the compliance landscape through precise device listings enables innovators to navigate the challenges of introducing new products more effectively.
Bioaccess® accelerates approval processes to just 6-8 weeks, significantly faster than the typical 6-12 months seen in the US and EU. This expedited method allows startups to enroll treatment-naive cardiology or neurology cohorts 50% quicker than their Western counterparts, facilitating faster market access and enhancing their competitive edge.
This streamlined approach not only aids startups in overcoming compliance hurdles but also promotes collaboration between oversight agencies and innovators. For instance, Welwaze Medical Inc. has partnered with bioaccess™ to successfully launch the Celbrea® medical product in Colombia, illustrating how strategic partnerships can lead to the development of innovative medical technologies that enhance patient care.
Cooperation among stakeholders—including manufacturers, regulatory authorities, and healthcare providers—is paramount for the development of effective device listings. By collaborating, these stakeholders can guarantee that listings are not only comprehensive and accurate but also reflective of the latest industry standards. The Clinical Research Management Expert emphasizes that fostering open communication and collaboration can yield improved outcomes for all parties involved. This collaborative effort ultimately enhances patient care and safety, underscoring the critical nature of teamwork in the clinical research landscape.
Effective device listings are paramount to achieving clinical success, ensuring compliance, enhancing credibility, and facilitating market access for medical technologies. This article underscores that a strategic approach to device listings not only streamlines regulatory processes but also fosters innovation and collaboration among stakeholders in the healthcare sector.
Key benefits such as regulatory compliance, improved patient safety, and the ability to leverage market intelligence are critical in this discussion. Accurate device listings mitigate risks associated with non-compliance, enhance trust among healthcare providers and patients, and provide valuable insights for strategic decision-making. Furthermore, these listings are essential for optimizing reimbursement processes, ensuring that medical technologies reach patients promptly and efficiently.
In conclusion, the importance of effective device listings cannot be overstated. Organizations must prioritize accurate and up-to-date listings to navigate the complexities of regulatory frameworks, foster innovation, and ultimately enhance patient care. By embracing these practices, companies can secure a competitive edge and contribute to the overall advancement of medical technology and its impact on healthcare outcomes.
What is bioaccess® and how does it help in medical device approvals?
bioaccess® is a consulting firm that utilizes its expertise in regulatory frameworks across Latin America, the Balkans, and Australia to expedite medical product approvals. It ensures precise and compliant product entries, significantly reducing the time needed to introduce new medical technologies, often obtaining ethical approvals in just 4-6 weeks.
Why is regulatory compliance important for medical equipment?
Regulatory compliance is crucial for the successful promotion of medical equipment as it ensures that device listings provide all necessary information for market entry. Maintaining accurate records helps mitigate risks associated with non-compliance, such as penalties and product recalls.
What are the anticipated changes in regulatory compliance by 2025?
By 2025, non-compliance ramifications are expected to become more severe, with increased scrutiny leading to a higher likelihood of recalls. Regular evaluations of equipment records are recommended to ensure adherence and facilitate a smoother approval process.
How do validated device listings enhance trust in medical devices?
Validated device listings serve as proof of the quality and safety of medical devices. When devices are listed with credible authorities like INVIMA, their reputation among healthcare providers, patients, and investors improves significantly, fostering trust in their safety and efficacy.
What role does INVIMA play in the regulatory process?
INVIMA, the Colombia National Food and Drug Surveillance Institute, is responsible for inspecting and supervising health products. It is classified as a Level 4 health authority by PAHO/WHO, highlighting the importance of regulatory compliance in building trust in medical devices.
Can you provide an example of a company using bioaccess® for market entry?
Welwaze Medical Inc. has engaged bioaccess® as its regulatory and market access consulting firm to assist in the introduction of the Celbrea® medical product in Colombia, demonstrating the importance of device listings for successful market integration.