


The article provides essential insights into ISO 13485:2016, underscoring its vital role in ensuring compliance for medical device manufacturers while enhancing product safety and effectiveness. Achieving ISO 13485 certification not only streamlines regulatory processes but also strategically positions organizations within the competitive global market. This is particularly evident given the rising demand for compliance in response to evolving industry standards and the necessity for robust quality management systems.
The medical technology landscape is evolving rapidly, yet a staggering 82% of companies still struggle to achieve compliance with ISO 13485:2016 standards. This certification is not merely a regulatory checkbox; it represents a critical framework for ensuring quality management in medical devices. As the industry anticipates a shift toward stricter regulations by 2025, understanding the nuances of ISO 13485 compliance becomes paramount.
What challenges do organizations face in navigating this complex landscape? How can they leverage the latest insights to not only comply but thrive in a competitive market? The answers to these questions are crucial for organizations aiming to enhance their operational effectiveness and ensure product quality.
bioaccess® excels in accelerating compliance with ISO 13485 2016 for Medtech innovators by leveraging its extensive understanding of regulatory frameworks across Latin America, the Balkans, and Australia. This strategic approach streamlines the approval process, enabling companies to achieve compliance in significantly less time than the industry average—ranging from 3 to 6 months for smaller organizations and 8 to 12 months for larger ones. Such efficiency is particularly advantageous for startups and small enterprises, which often face resource constraints when navigating complex regulatory landscapes. By facilitating faster compliance, bioaccess® not only accelerates time to market but also enhances the overall marketability of innovative medical devices, positioning clients for success in a competitive environment.
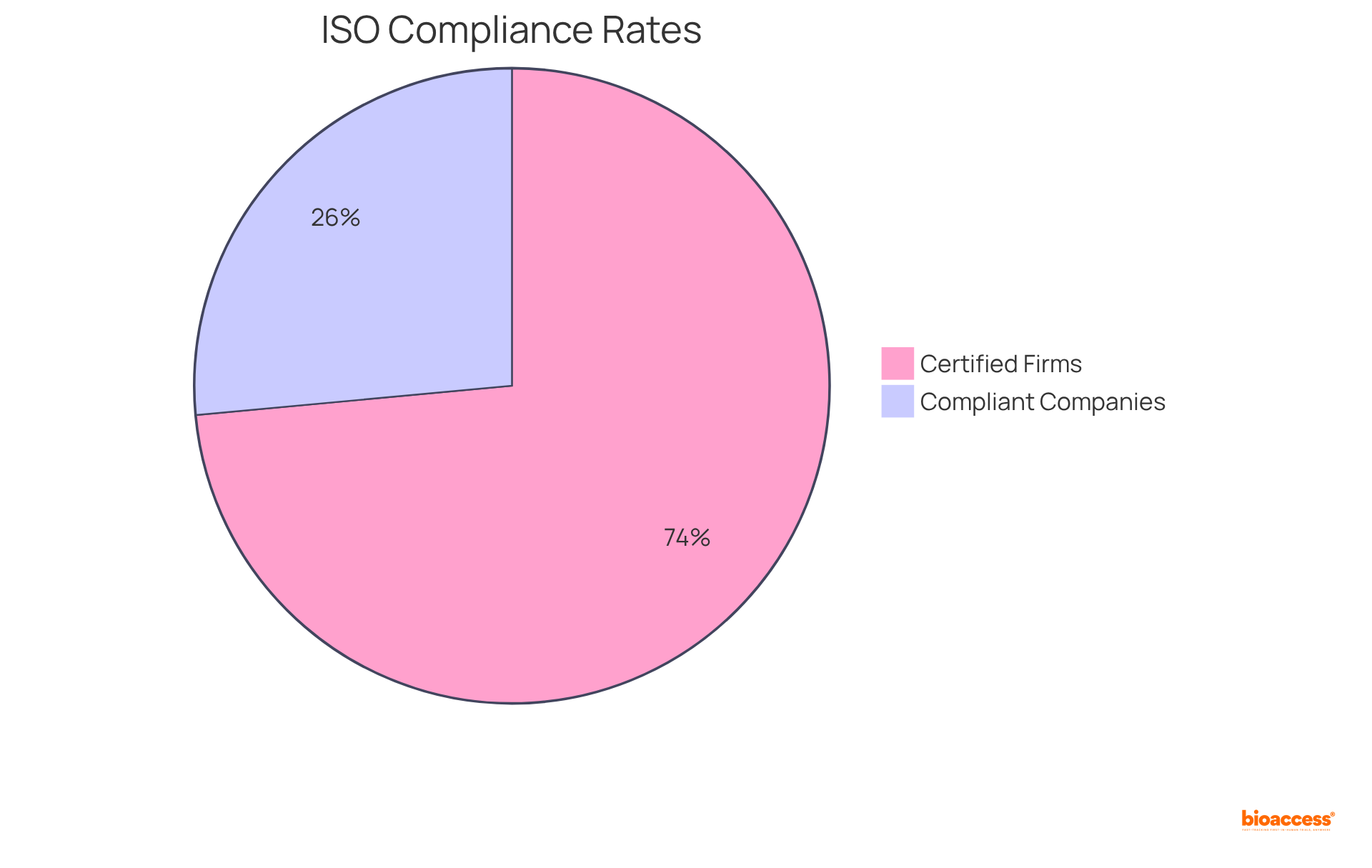
With only 18% of companies currently compliant with ISO regulations and 50% of medical device firms certified to ISO 13485 2016, the demand for effective strategies is critical. Furthermore, organizations must demonstrate that their administration systems have been functioning for at least three months prior to certification. As the ISO 13485 2016 certification is anticipated to align with the FDA's Quality System Regulation (QSR) by December 2024, bioaccess® stands ready to assist Medtech companies in this vital effort.
ISO 9001 represents the global benchmark outlining criteria for a Quality Management System (QMS) in the medical device sector. It ensures that organizations consistently meet customer and regulatory requirements, thereby enhancing product safety and effectiveness. For producers aiming to demonstrate their commitment to excellence and gain access to international markets, adherence to ISO standards is essential. This standard emphasizes a process-oriented approach, focusing on risk management and continuous improvement throughout the product lifecycle. Certification remains valid for three years, necessitating annual surveillance audits to sustain compliance.
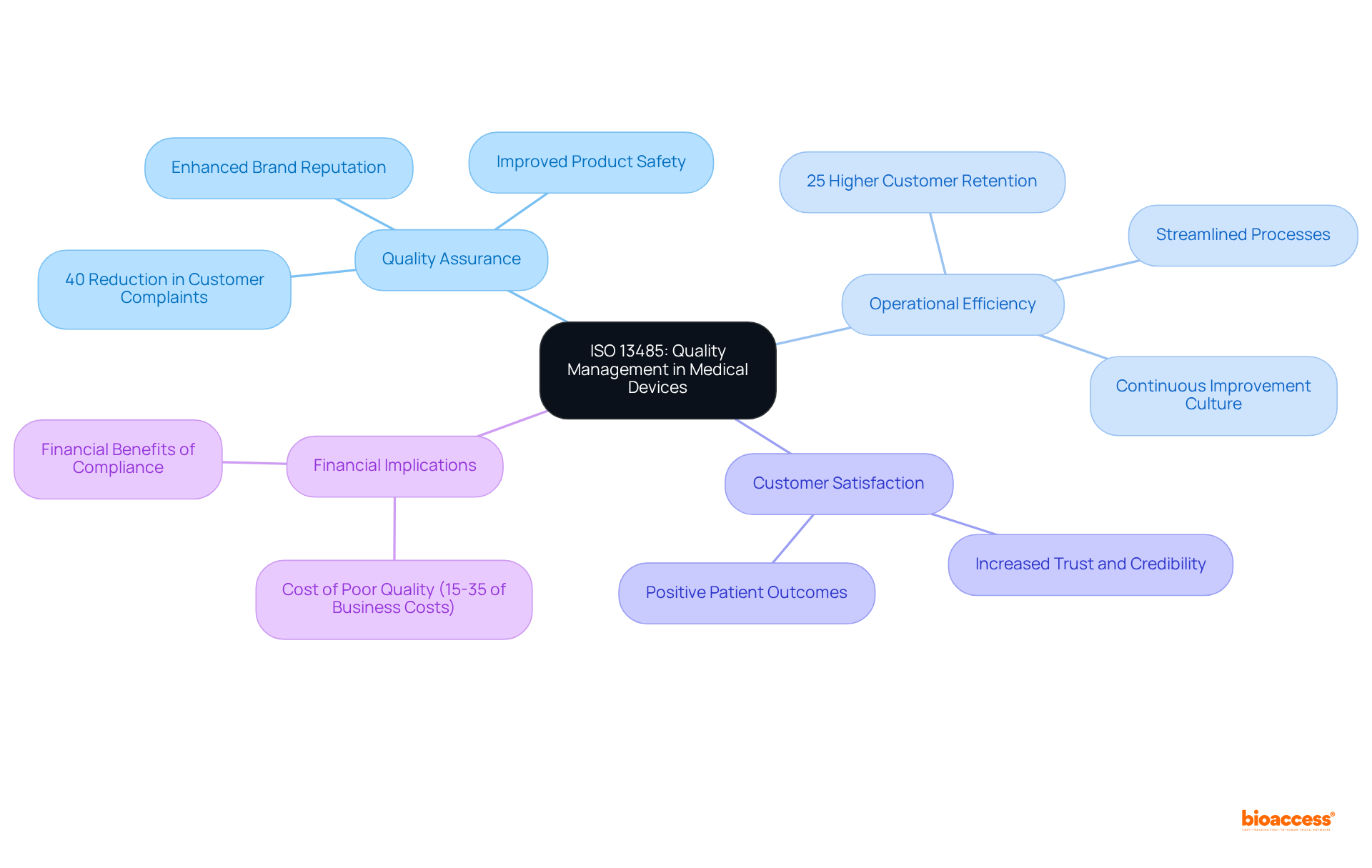
Quality assurance specialists assert that compliance with ISO standards fosters a culture of continuous improvement, enabling organizations to enhance operational efficiencies and product standards. Companies implementing effective QMS practices have reported a 40% reduction in customer complaints, illustrating the tangible benefits of compliance. Furthermore, organizations with efficient management systems experience a 25% higher customer retention rate, as noted by the Aberdeen Group.
Real-world examples illustrate how compliance with ISO standards has significantly improved product safety in medical devices. During the COVID-19 pandemic, the critical role of certified devices became evident, as producers with ISO certification were better positioned to ensure standards and compliance, ultimately enhancing patient safety.
The benefits of ISO compliance extend beyond regulatory adherence; they include improved brand reputation and increased customer trust. Organizations achieving certification are often perceived as reputable and trustworthy, leading to enhanced business opportunities. The financial implications of inadequate products in regulated sectors can range from 15% to 35% of total business expenses, underscoring the costs associated with failing to meet performance benchmarks.
As the Medtech landscape evolves in 2025, the importance of ISO compliance will only grow. Manufacturers must understand that compliance is not merely a regulatory obligation but a strategic advantage that can facilitate market access and operational excellence. By embracing the ISO standard, companies can position themselves as leaders in quality oversight, ensuring their products meet the highest safety and effectiveness standards.
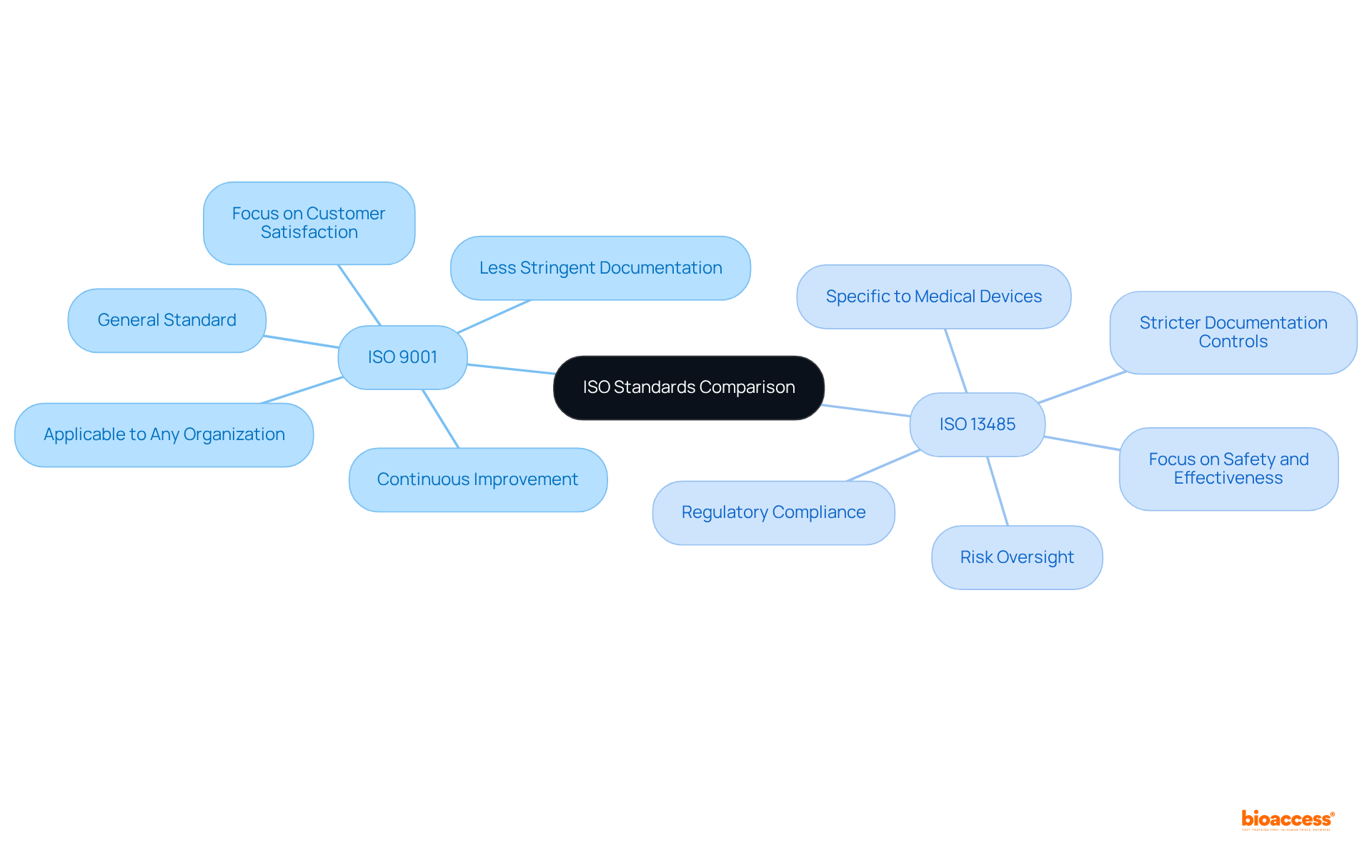
Both ISO 9001 and ISO 13485 are frameworks designed for excellence, yet they fulfill distinct roles. ISO 9001 serves as a general standard applicable to any organization, with a primary focus on customer satisfaction and continuous improvement. In contrast, ISO 13485:2016 is tailored specifically for the medical device sector, placing a strong emphasis on regulatory compliance and risk oversight. This standard necessitates that organizations document their processes in greater detail and maintain a higher level of control over product standards, making it essential for Medtech companies.
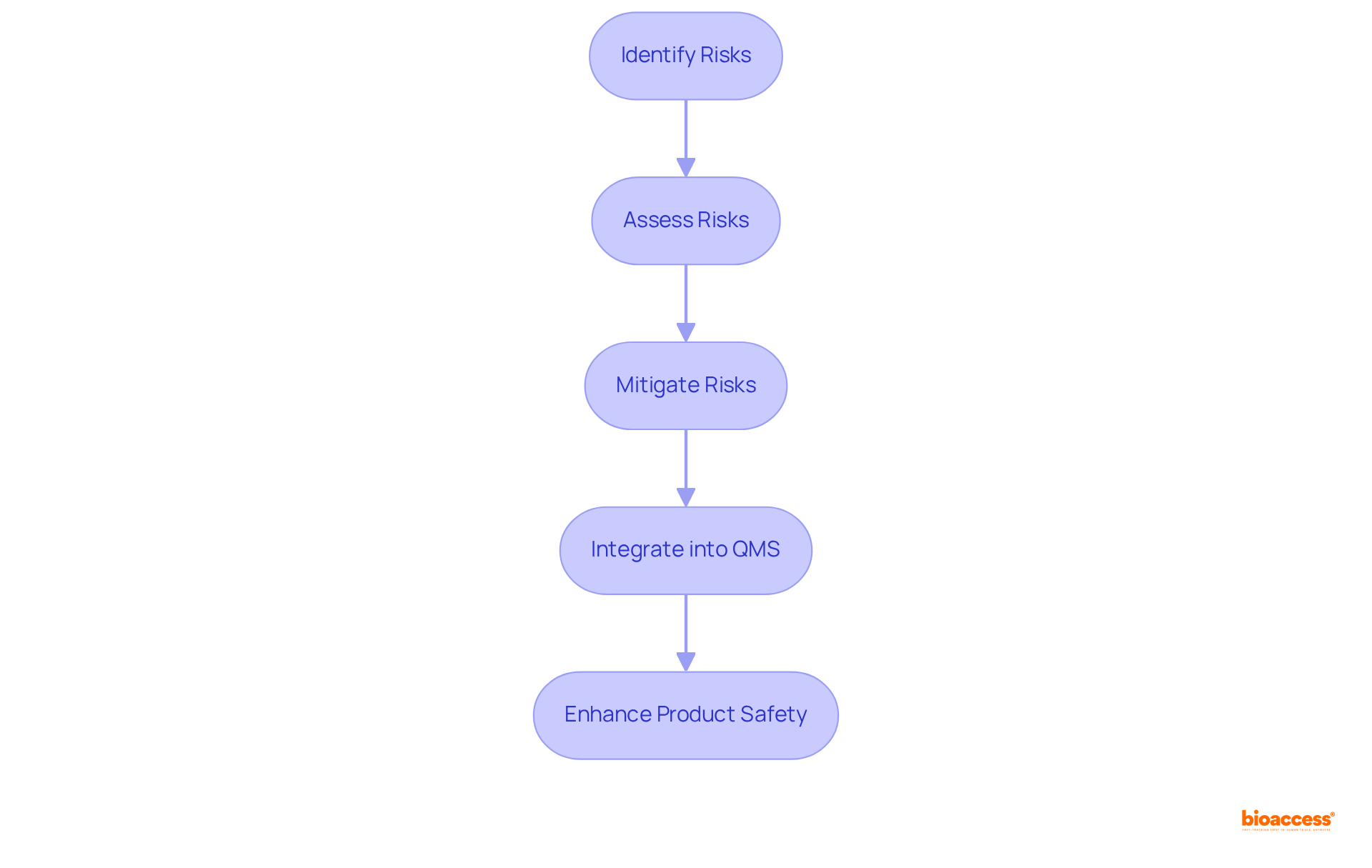
The 2016 revision to the ISO 13485 2016 standards introduced a risk-oriented strategy aimed at enhancing the standards' effectiveness. Organizations are now required to recognize and mitigate risks throughout the product lifecycle. This significant shift underscores the importance of proactive risk oversight over reactive measures, enabling companies to anticipate potential issues before they arise. By embedding risk assessment into their Quality Management Systems (QMS) aligned with ISO 13485 2016, Medtech organizations can significantly enhance product safety, ensure compliance, and cultivate a culture of continuous improvement.
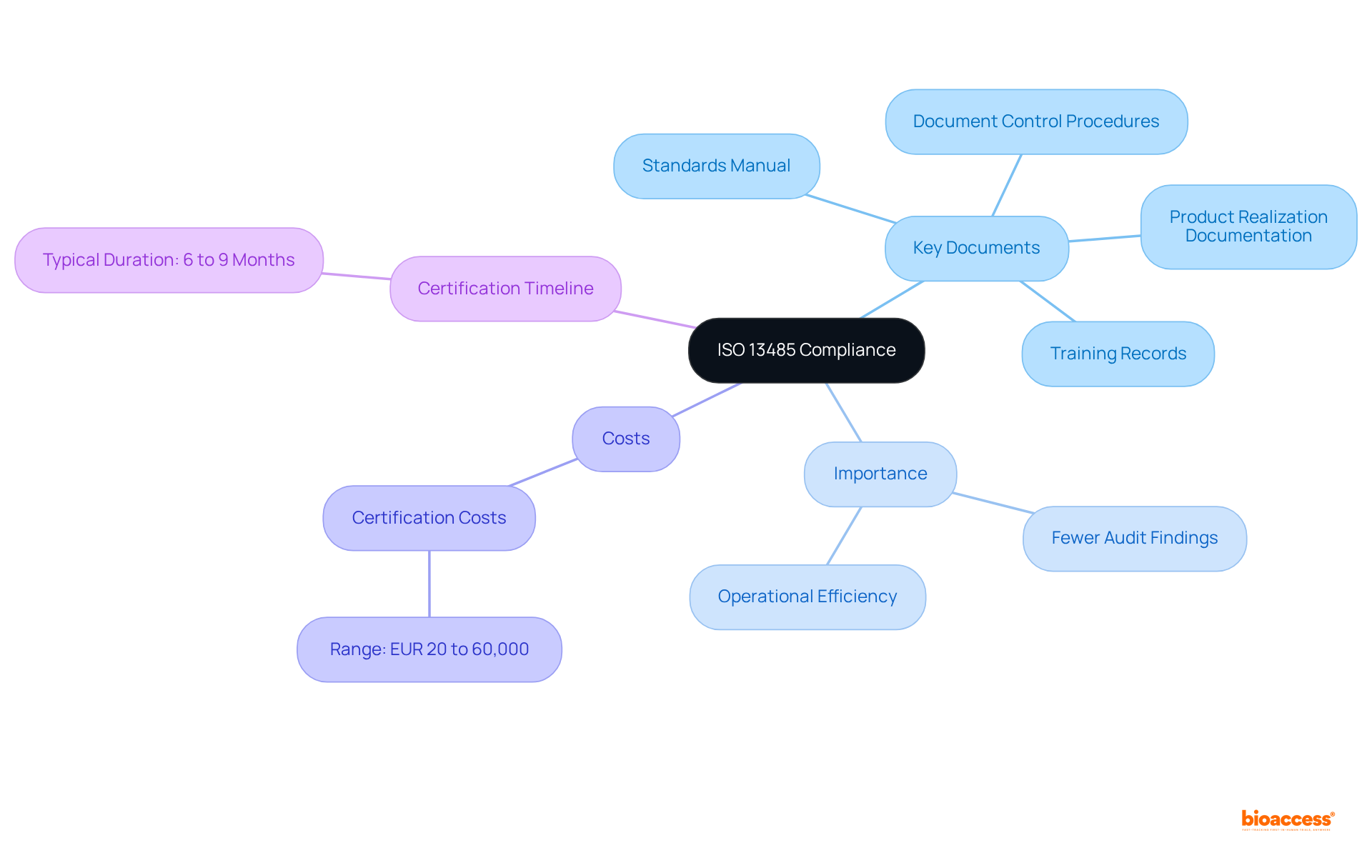
ISO 13485 2016 mandates comprehensive documentation to facilitate effective management of standards. Key documents include:
Organizations are also required to maintain records of nonconformities and the corrective actions taken. This documentation not only supports compliance with ISO 13485 2016 but also aids audits and inspections, providing evidence of conformity to ISO 13485 2016 standards. Statistics indicate that organizations with well-organized documentation practices experience significantly fewer audit findings.
Furthermore, external certification costs for quality control systems range from EUR 20 to 60,000, highlighting the financial benefits of efficient documentation. Implementing a hierarchical documentation structure, as illustrated in the case study on QMS Documentation Structure, improves clarity and consistency, further bolstering compliance and operational efficiency within the Medtech industry.
Moreover, the process of obtaining ISO 13485 2016 certification typically spans six to nine months, making timely and systematic documentation crucial for organizations aiming for compliance with ISO 13485 2016.
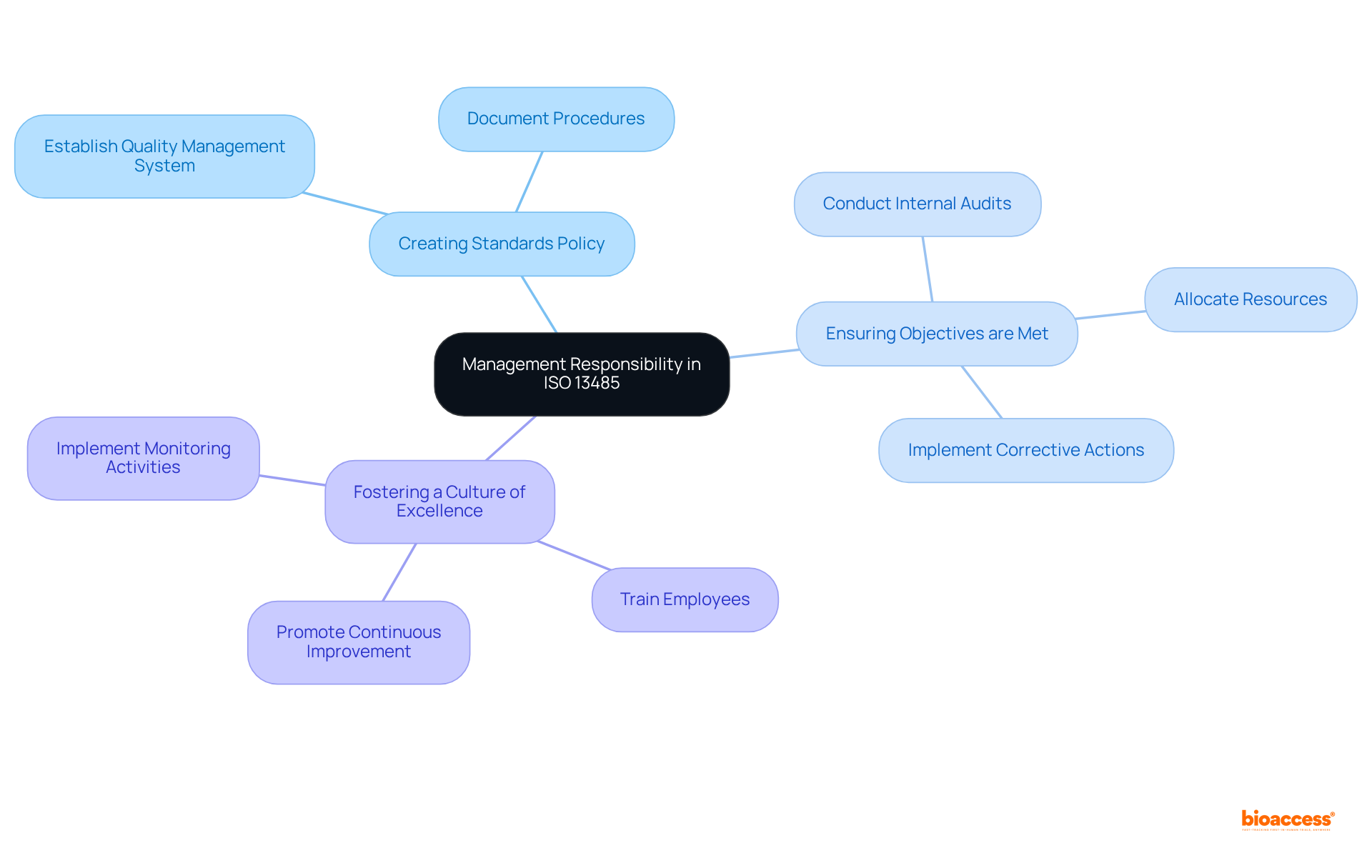
Top executives play a pivotal role in the successful implementation of ISO 13485, organized into eight main clauses. They are accountable for:
Management must allocate adequate resources for the Quality Management System (QMS) and ensure that employees are trained and competent. As Margo Barr states, "Quality Management Operations In the realm of medical devices, ensuring safety and efficacy is not just a priority—it is imperative." This dedication to excellence by leadership significantly influences the overall efficiency of the QMS and promotes ongoing enhancement, which is essential for compliance with ISO 13485 2016.
Furthermore, organizations should implement monitoring activities for products and processes to showcase leadership’s commitment to excellence. As William Foster aptly puts it, "Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution." This underscores the necessity of intentional leadership in the management of standards.
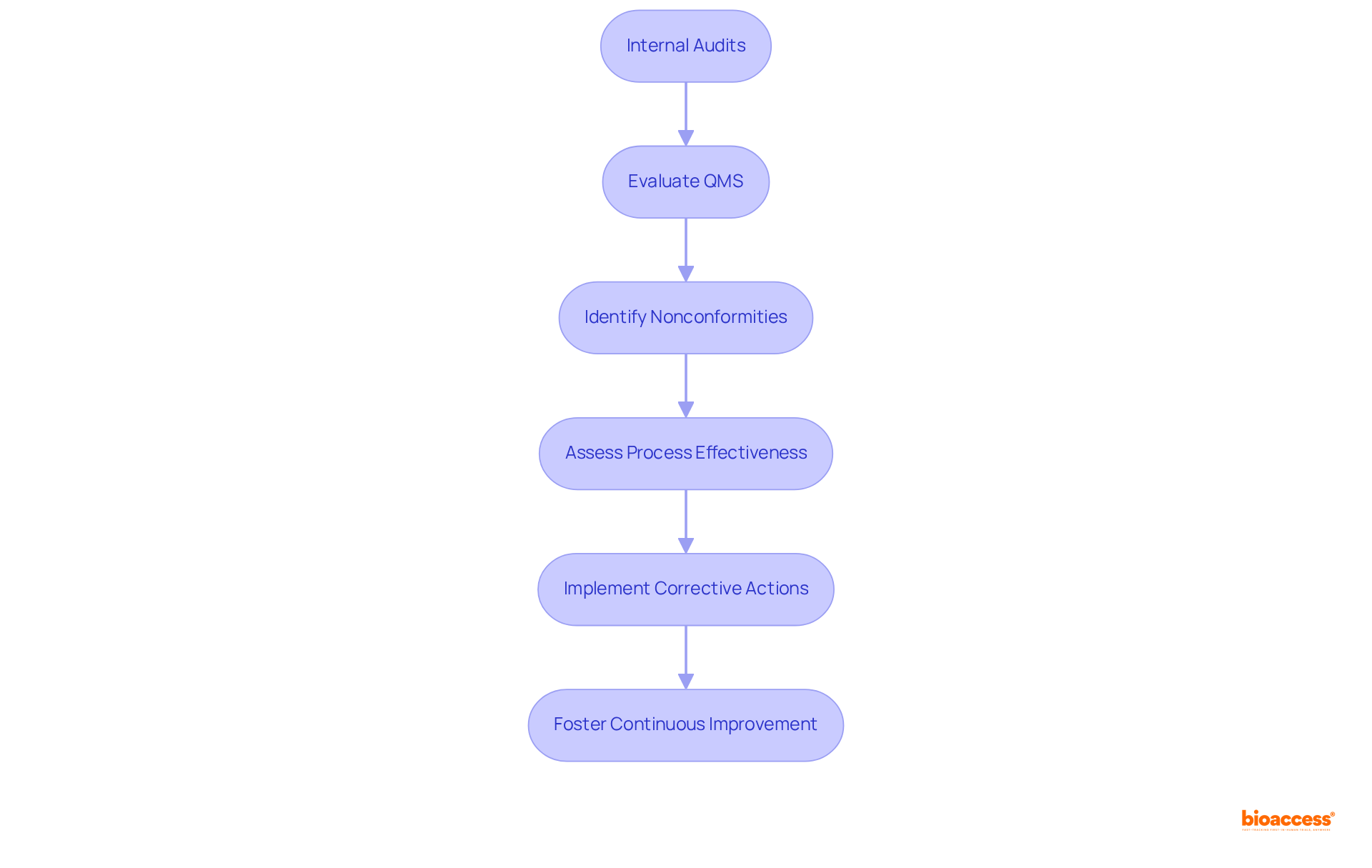
Internal audits are essential for achieving ISO compliance, acting as a systematic evaluation of the Quality Management System (QMS) to ensure adherence to the standard's requirements. These audits are instrumental in identifying nonconformities, evaluating process effectiveness, and enabling timely corrective actions. Regular internal audits foster a culture of accountability and drive continuous improvement, which is vital for sustaining compliance and enhancing product quality.
Research reveals that organizations conducting internal audits quarterly are 40% more likely to pass official inspections without significant findings, highlighting the necessity of a proactive audit strategy. Moreover, effective internal audit practices, such as performing comprehensive gap analyses and involving cross-functional teams, can markedly enhance audit readiness and compliance rates.
Regulatory experts assert that a well-structured audit process is crucial for ensuring that the QMS is not only established but also effectively maintained, ultimately resulting in superior product outcomes and market success. Furthermore, ISO standard clause 8.2.4 mandates internal audits, underscoring their significance for compliance. Organizations must also implement a methodology for addressing corrective actions to establish an effective CAPA system, as neglecting these practices can lead to severe consequences, including restricted market opportunities.
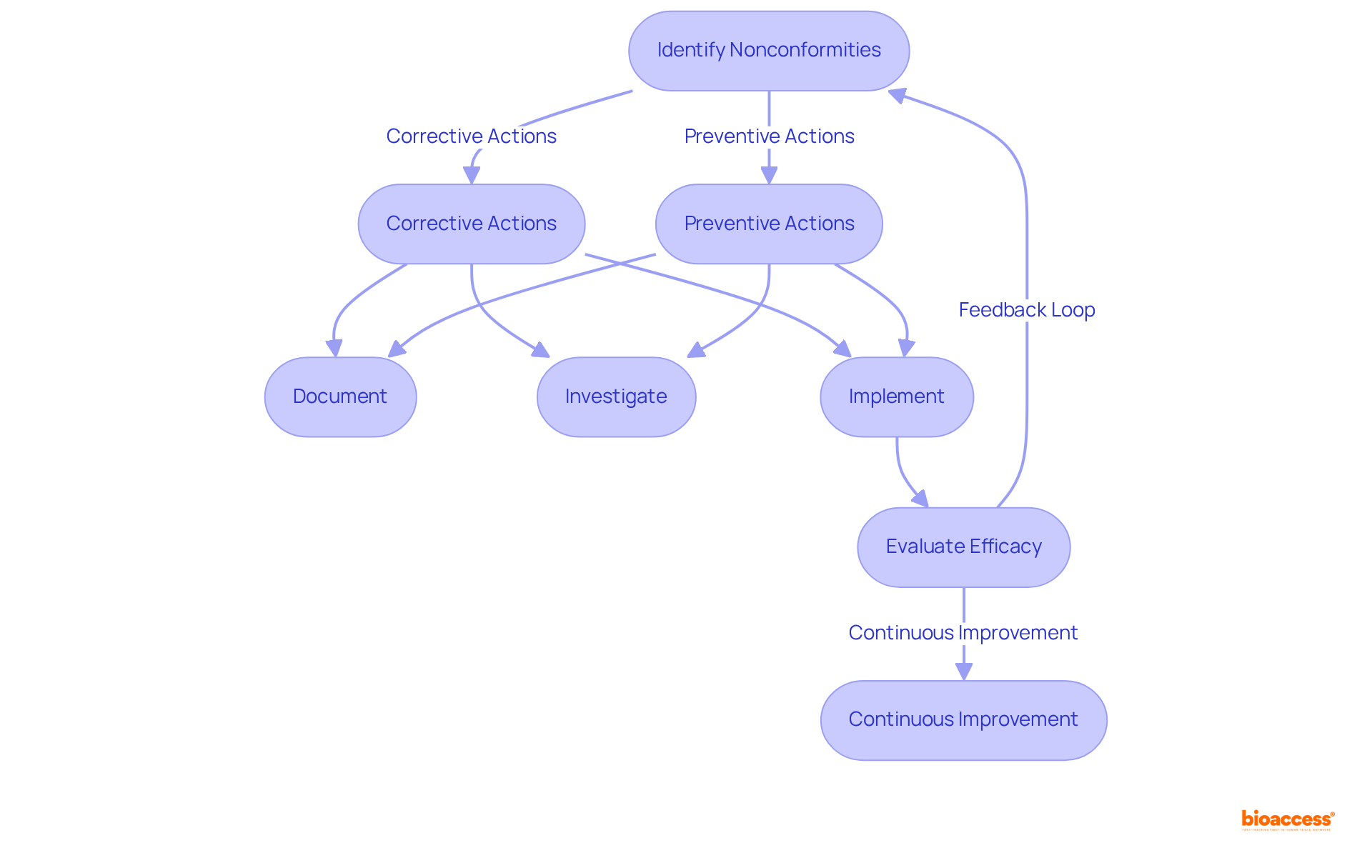
ISO standards underscore the critical importance of efficient corrective and preventive actions (CAPA) in addressing nonconformities and preventing their recurrence. Organizations must establish robust procedures for identifying, documenting, and investigating nonconformities, alongside implementing corrective actions to mitigate their impact. Preventive actions are equally vital, aimed at eliminating potential causes of nonconformities before they manifest. Effective CAPA oversight not only enhances the Quality Assurance System (QAS) but also fosters ongoing improvements in product standards.
In 2016, nearly 50% of all FDA 483 letters issued were related to CAPA failures, emphasizing the urgent need for organizations to refine their CAPA processes. The FDA has intensified inspections at medical device manufacturing facilities by 46% since 2007, with foreign facility inspections soaring by 243% during the same timeframe. This heightened scrutiny highlights the necessity of maintaining a proactive approach to CAPA, as organizations frequently encounter challenges such as inadequate root cause determination and a reactive mindset.
Upcoming updates to ISO standards, particularly in 2025, will transition to a more integrated CAPA framework, aligning with the Medical Device Single Audit Program (MDSAP). This transition requires organizations not only to document their CAPA procedures but also to ensure that they are effectively followed and evaluated for efficacy. The Average Resolution Time KPI, which measures the duration taken to resolve CAPAs, acts as a crucial indicator of process efficiency. A prolonged resolution time may indicate inefficiencies in root cause analysis or action implementation, while a shorter duration reflects a well-managed CAPA process.
Insights from excellence specialists underscore the importance of a structured approach to CAPA. For example, maintaining a 'lessons learned' database can significantly enhance the identification and resolution of recurring issues, as illustrated in a case study where such a database led to improved CAPA processes. Moreover, organizations are encouraged to embrace a Kaizen approach, advocating for small, continuous changes that drive productivity improvements.
By prioritizing efficient CAPA oversight, organizations can not only comply with the ISO 13485 2016 standards but also enhance their overall assurance initiatives, ultimately leading to improved patient outcomes and reduced costs associated with failures in excellence.
The ISO standard has experienced significant evolution since its initial publication in 1996, reflecting the dynamic nature of the medical device industry and the necessity for alignment with international regulations. The revision of ISO 13485 2016 marked a pivotal shift, introducing a risk-based approach that prioritizes patient safety and regulatory compliance. This update emphasized the critical need for thorough documentation and increased oversight responsibility, ensuring organizations uphold a comprehensive quality management system (QMS). Notably, companies must possess their ISO 13485 2016 certificate by January 1, 2019, to sell in Canada, highlighting the urgency for compliance within the Medtech sector.
Organizations adapting to these changes have reported improved compliance practices and enhanced operational efficiency. For instance, producers adopting a phased strategy for ISO certification have discovered that the organized development of their QMS not only facilitated regulatory compliance but also elevated product standards and customer satisfaction.
The updates to ISO have prompted Medtech firms to reevaluate their quality control strategies. Regular audits and management reviews, now mandated under the ISO 13485 2016 standard, have become essential for identifying emerging issues and ensuring continuous improvement. As industry expert Jon Speer notes, "Your ISO auditor is going to want to see your gap analysis. That is going to be a key artifact as part of this transition process," highlighting the importance of this tool in the transition.
Furthermore, the alignment of ISO standards with European medical device directives has fortified its position as a foundation for adherence in the medical device sector. Regulatory bodies emphasize that compliance with ISO standards not only enhances product safety but also cultivates trust among stakeholders, ultimately leading to increased market opportunities. As the landscape of medical device regulation continues to evolve, staying informed about these changes is vital for organizations aiming to thrive in a competitive environment.
ISO standards are recognized worldwide as the benchmark for management systems within the medical device sector. Compliance with these standards not only signifies a dedication to quality but also opens doors to international markets. Regulatory authorities in numerous countries frequently mandate ISO 13485:2016 certification as a critical component of the medical device approval process. By adhering to this globally respected standard, organizations can significantly bolster their credibility and enhance their competitiveness in the global marketplace.
ISO 13485:2016 stands as a fundamental pillar for quality management within the medical device industry, establishing a robust framework that ensures not only regulatory compliance but also enhances product safety and efficacy. As the Medtech landscape evolves, the imperative of adhering to this standard intensifies, equipping organizations to effectively tackle both current and future challenges.
This article underscores vital insights, including:
With only a fraction of companies presently compliant, the urgency for effective strategies is unmistakable. By emphasizing risk management and continuous improvement, ISO 13485:2016 not only facilitates regulatory compliance but also propels operational excellence, ultimately resulting in improved patient outcomes.
As Medtech companies strategize for the future, embracing ISO 13485 compliance transcends mere regulatory obligation; it emerges as a strategic advantage that can significantly bolster marketability and competitiveness. By prioritizing adherence to this globally recognized standard, organizations can ensure their products meet the highest safety and quality benchmarks, fostering trust among stakeholders and paving the way for success in an industry characterized by rapid change.
What is bioaccess® and how does it help Medtech companies?
bioaccess® specializes in accelerating compliance with ISO 13485:2016 for Medtech innovators by leveraging its understanding of regulatory frameworks across various regions. It streamlines the approval process, enabling companies to achieve compliance in 3 to 6 months for smaller organizations and 8 to 12 months for larger ones, which is significantly faster than the industry average.
Why is ISO 13485 compliance important for Medtech companies?
ISO 13485 compliance is crucial as it ensures that organizations meet customer and regulatory requirements, enhancing product safety and effectiveness. It also positions companies for success in competitive markets by improving marketability and reducing time to market for innovative medical devices.
What percentage of companies are currently compliant with ISO regulations?
Only 18% of companies are currently compliant with ISO regulations, and 50% of medical device firms are certified to ISO 13485:2016.
What are the requirements for ISO 13485 certification?
Organizations must demonstrate that their administration systems have been functioning for at least three months prior to certification.
How does ISO 13485 certification relate to the FDA's Quality System Regulation (QSR)?
The ISO 13485:2016 certification is anticipated to align with the FDA's Quality System Regulation (QSR) by December 2024.
What are the benefits of ISO 9001 and ISO 13485 compliance?
Compliance with ISO standards fosters a culture of continuous improvement, enhances operational efficiencies, and improves product standards. Companies implementing these practices have reported a 40% reduction in customer complaints and a 25% higher customer retention rate.
How does ISO compliance impact product safety?
Compliance with ISO standards has been shown to significantly improve product safety in medical devices, as demonstrated during the COVID-19 pandemic when certified devices ensured higher standards and compliance, enhancing patient safety.
What are the financial implications of non-compliance in regulated sectors?
The financial implications of inadequate products in regulated sectors can range from 15% to 35% of total business expenses, highlighting the costs associated with failing to meet performance benchmarks.
What is the difference between ISO 9001 and ISO 13485?
ISO 9001 is a general standard focused on customer satisfaction and continuous improvement applicable to any organization. In contrast, ISO 13485:2016 is specifically tailored for the medical device sector, emphasizing regulatory compliance and risk oversight, requiring more detailed documentation and control over product standards.