


The landscape of post-approval monitoring for biologics in Australia is rapidly evolving, driven by a steadfast commitment to patient safety and regulatory compliance. As the demand for innovative biologic therapies continues to rise, grasping the intricacies of monitoring practices becomes essential for healthcare stakeholders.
What challenges lie ahead in ensuring that these therapies remain safe and effective? How can collaborative efforts among industry players enhance oversight?
This article delves into key insights on post-approval monitoring of biologics in Australia, exploring strategies, innovations, and the critical role of stakeholder engagement in shaping future practices.
bioaccess® is at the forefront of post-approval monitoring of biologics in Australia, utilizing its extensive clinical research expertise to uphold compliance and quality standards. With a strategic presence across Latin America, the Balkans, and Australia, bioaccess® enhances the evaluation process, facilitating quicker identification of alert signals and efficacy information. This agility is vital in today’s ever-changing regulatory landscape, ensuring that the post-approval monitoring of biologics in Australia consistently meets health standards.
Industry leaders emphasize that robust adherence to post-approval monitoring of biologics in Australia is crucial for maintaining public trust and ensuring safety for individuals. Successful strategies employed by bioaccess® include:
These initiatives not only protect patient health but also foster the ongoing development of innovative biologics in Australia.
As the Medtech landscape evolves, bioaccess® plays a pivotal role in addressing key challenges, reinforcing the importance of collaboration among stakeholders. By prioritizing post-approval monitoring of biologics in Australia, bioaccess® not only safeguards public health but also drives the advancement of biologics, ensuring their safety and effectiveness for all.
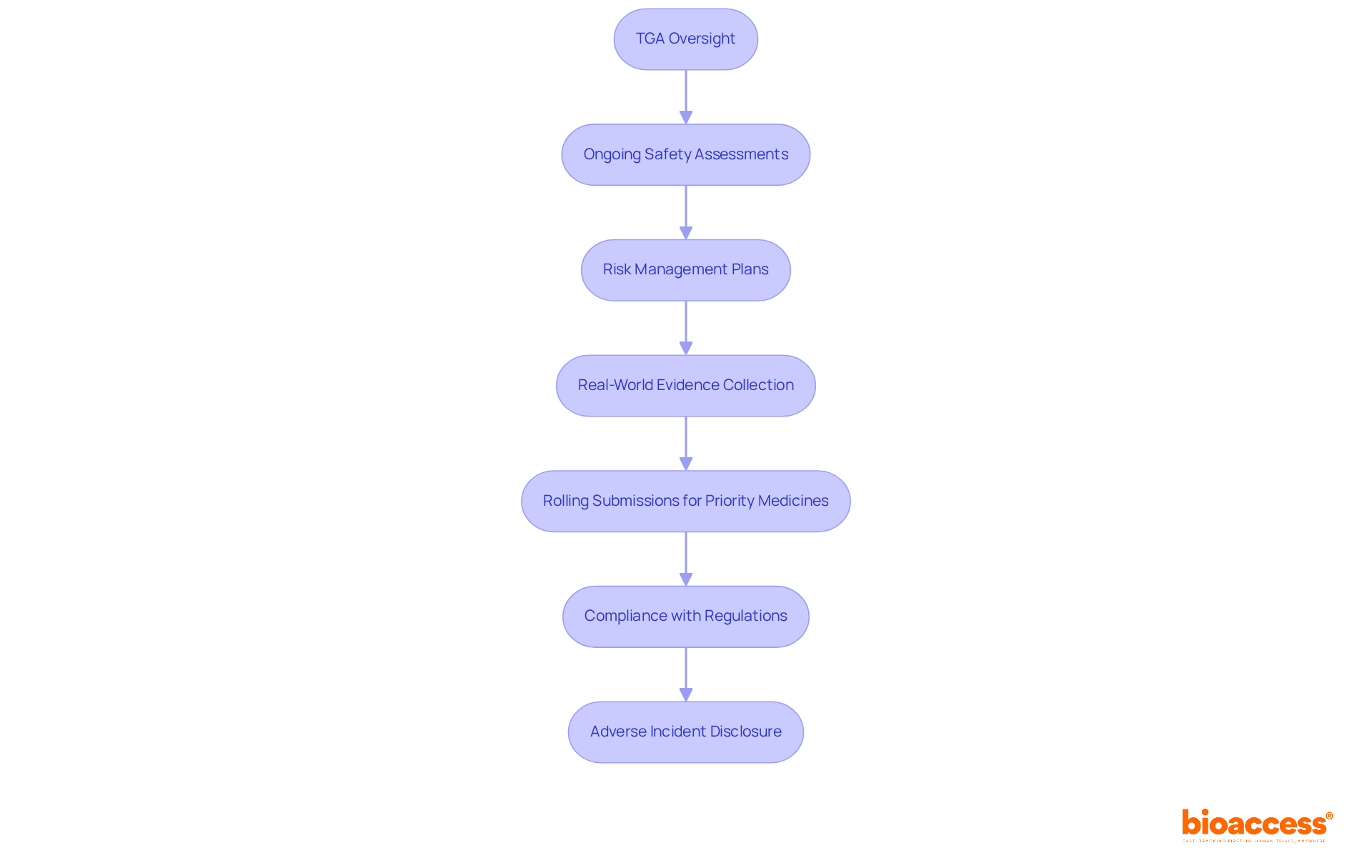
The Therapeutic Goods Administration (TGA) primarily oversees the Australian regulatory structure for biologics, having established comprehensive guidelines for post-approval monitoring of biologics in Australia. These guidelines highlight the critical need for ongoing safety assessments, robust risk management plans, and the systematic collection of real-world evidence. This ensures that biologics uphold their safety and efficacy throughout their lifecycle.
In 2025, the TGA introduced rolling submissions for priority medicines, allowing for timely data submission as it becomes available. This innovation significantly enhances the monitoring process. Compliance with these regulations is vital, not only for protecting public health but also for fostering trust among patients and healthcare professionals. Recent updates indicate that the TGA aims to complete the examination of applications for biologics and biosimilars within 255 working days, reflecting a strong commitment to efficiency in regulatory processes.
Moreover, manufacturers are required to disclose any adverse incidents they encounter, ensuring that critical information is communicated and addressed swiftly. This continuous vigilance, including post-approval monitoring of biologics in Australia, is essential for identifying potential risk signals and implementing necessary regulatory actions, such as updating product labels or issuing warnings. Such measures reinforce the TGA's pivotal role in maintaining the integrity of the healthcare system.
Post-approval monitoring of biologics in Australia is crucial for ensuring ongoing safety and effectiveness in clinical research. This process involves the systematic collection and analysis of diverse data types, including:
Key metrics, such as incidence rates of adverse reactions and patient-reported outcomes, are essential for evaluating the continuous safety profile of biologics.
Recent data reveals that:
Such insights are vital for informing regulatory decisions and guiding clinical practice, especially regarding post-approval monitoring of biologics in Australia.
Moreover, incorporating real-world evidence, including outcomes data, enhances our understanding of the long-term effects of biologics. This ultimately aids in developing better management and treatment strategies. Promoting documentation and collaboration between physicians and pharmacists is essential to prevent, monitor, and treat biopharmaceutical adverse drug reactions (ADRs). By fostering these partnerships, we can ensure a more effective approach to patient care.
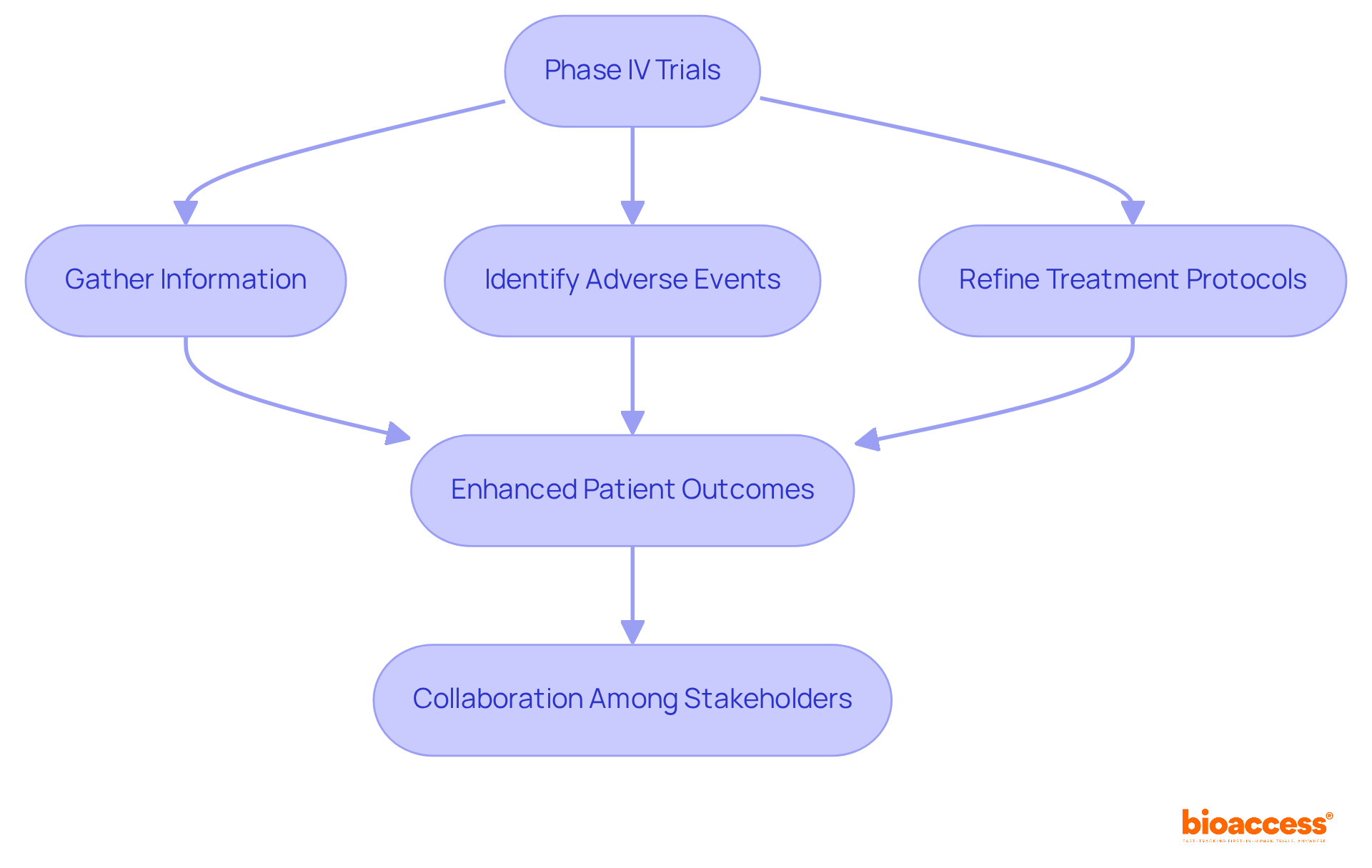
Clinical trials are essential for post-approval monitoring of biologics in Australia. Phase IV trials, also known as post-marketing studies, are designed to gather additional information about a product's reliability, efficacy, and optimal use across a broader patient population. These trials play a crucial role in identifying rare adverse events and long-term effects that may not have been evident during pre-approval studies. By doing so, they enhance the overall risk profile of biologics, ensuring that these products remain safe and effective for patients.
The insights gained from Phase IV trials are invaluable. They not only contribute to the ongoing assessment of biologics but also help in refining treatment protocols and improving patient outcomes. As the Medtech landscape evolves, the importance of these studies cannot be overstated. They serve as a foundation for informed decision-making and foster trust in biologic therapies.
In conclusion, collaboration among stakeholders in clinical research is vital. By prioritizing post-approval monitoring of biologics in Australia, we can address key challenges and ensure that biologics continue to effectively meet the needs of patients. The next steps involve leveraging the findings from these trials to enhance clinical practices and patient safety.
Patient feedback is crucial in the post-approval monitoring of biologics in Australia, providing essential insights into treatment effectiveness and tolerability in real-world settings. Engaging individuals through methods like surveys, focus groups, and registries allows for the collection of valuable data that significantly enhances ongoing safety assessments. For instance, the American Academy of Orthopaedic Surgeons (AAOS) has initiated the Orthobiologics Registry, starting with 10 participating sites, to monitor reported outcomes and long-term effectiveness of treatments. This initiative showcases a commitment to integrating patient experiences into clinical evaluations.
Moreover, incorporating insights from patients not only informs regulatory bodies but also guides healthcare providers in refining treatment protocols. This ultimately leads to improved care and outcomes. As the demand for innovative biologic therapies rises, the authentic involvement of patients becomes increasingly vital, ensuring that research and oversight processes reflect the needs and experiences of those directly affected by these treatments.
Notably, patient-engaged research achieves enrollment targets 25% faster and experiences 40% fewer protocol amendments. These statistics underscore the benefits of incorporating patient feedback in clinical research. As Dr. Maureen Bisognano aptly stated, "Patient engagement is not a nice-to-have in modern healthcare research - it's an ethical imperative and a scientific necessity." This highlights the urgent need for collaboration and the next steps in enhancing patient involvement in clinical research.

Technological innovations are revolutionizing the post-approval monitoring of biologics in Australia. By integrating real-world information analytics, artificial intelligence, and electronic health records, organizations can collect and analyze data more efficiently. This technological advancement not only facilitates the identification of safety signals but also supports the post-approval monitoring of biologics in Australia, revealing trends in patient outcomes and enabling timely interventions that significantly enhance patient safety.
However, the effectiveness of these innovations hinges on the quality of information. High-quality data is essential for precise oversight and informed decision-making. Moreover, fostering data literacy within organizations is crucial; it empowers teams to leverage data effectively for oversight purposes. Consider how your organization can improve its data practices to enhance monitoring capabilities.
Furthermore, digital platforms designed for user engagement simplify feedback collection and enhance communication among stakeholders. This streamlined interaction ultimately leads to better health outcomes. As we move forward, collaboration among all parties involved will be vital in harnessing these technological advancements to their fullest potential.
Collaboration among stakeholders - including regulatory agencies, healthcare providers, patients, and industry representatives - is crucial for effective post-approval oversight of biologics. This collaboration not only fosters strong communication pathways but also establishes cooperative structures that enhance data sharing. As a result, stakeholders can respond promptly to emerging concerns. Frequent meetings and collaborative projects nurture partnerships, while shared databases facilitate thorough oversight processes.
Successful collaborations have demonstrated that incorporating diverse stakeholder viewpoints leads to more effective evaluation results. This ultimately guarantees safety for individuals and ensures product effectiveness. Moreover, expert opinions underscore the necessity of continuous engagement and feedback mechanisms to adapt to the evolving challenges within the biologics landscape. By prioritizing collaboration, stakeholders can significantly enhance the post-approval oversight framework, proactively and efficiently addressing potential issues.
Post-approval monitoring of biologics in Australia is crucial in ensuring patient safety and regulatory compliance. However, this area faces significant challenges, including:
Alarmingly, statistics reveal that around 90% of adverse drug reactions (ADRs) go unreported. In Canada alone, approximately 200,000 ADRs are reported annually, with about 22,000 resulting in death. This stark reality underscores the urgent need for efficient oversight systems.
Limited resources and varying levels of stakeholder commitment can further hinder these efforts. To effectively tackle these obstacles, stakeholders must prioritize cooperation across sectors. Investing in advanced technology for information management and establishing standardized protocols for information collection and analysis, such as those outlined in the Safety Assessment Marketed Medicines (SAMM) guidelines launched in 1994, is essential. Ongoing training and education for all parties involved are vital to enhance the overall effectiveness of oversight initiatives.
For instance, the Yellow Card scheme in the UK, established in 1964 following the thalidomide tragedy, has successfully evolved to include electronic reporting. This evolution has significantly increased the volume of ADR reports and improved data quality. By learning from such examples, stakeholders can implement similar strategies to enhance post-approval oversight in Australia. Collaboration and commitment to these initiatives are imperative for improving patient safety and regulatory compliance.
The future of post-approval monitoring of biologics in Australia is poised for significant transformation, driven by technological advancements, regulatory evolution, and a heightened emphasis on patient-centered care. This evolution is not just a trend; it’s a necessary shift that will redefine how we approach clinical research.
The integration of real-world evidence (RWE) and advanced analytical techniques will enhance oversight capabilities, facilitating more proactive risk evaluations. For instance, the FDA's Sentinel Initiative exemplifies how real-time data can bolster pharmacovigilance by actively monitoring drug safety through diverse data sources. This approach not only improves our understanding of treatment impacts across various demographics but also aids in identifying potential risk issues earlier in the product lifecycle.
Regulatory bodies are expected to adopt more adaptable frameworks to enhance the post-approval monitoring of biologics in Australia that can keep pace with the rapid advancements in biologics. This ensures that oversight practices remain relevant and efficient, addressing the evolving landscape of healthcare. Specialists agree that leveraging RWE will be crucial in refining oversight practices, as it provides insights that traditional clinical trials might overlook. Ultimately, this leads to better health outcomes and more informed healthcare decisions.
As we navigate this transformative period, collaboration among stakeholders will be essential. By embracing these advancements, we can enhance the effectiveness of clinical research and ensure that patient safety remains at the forefront.
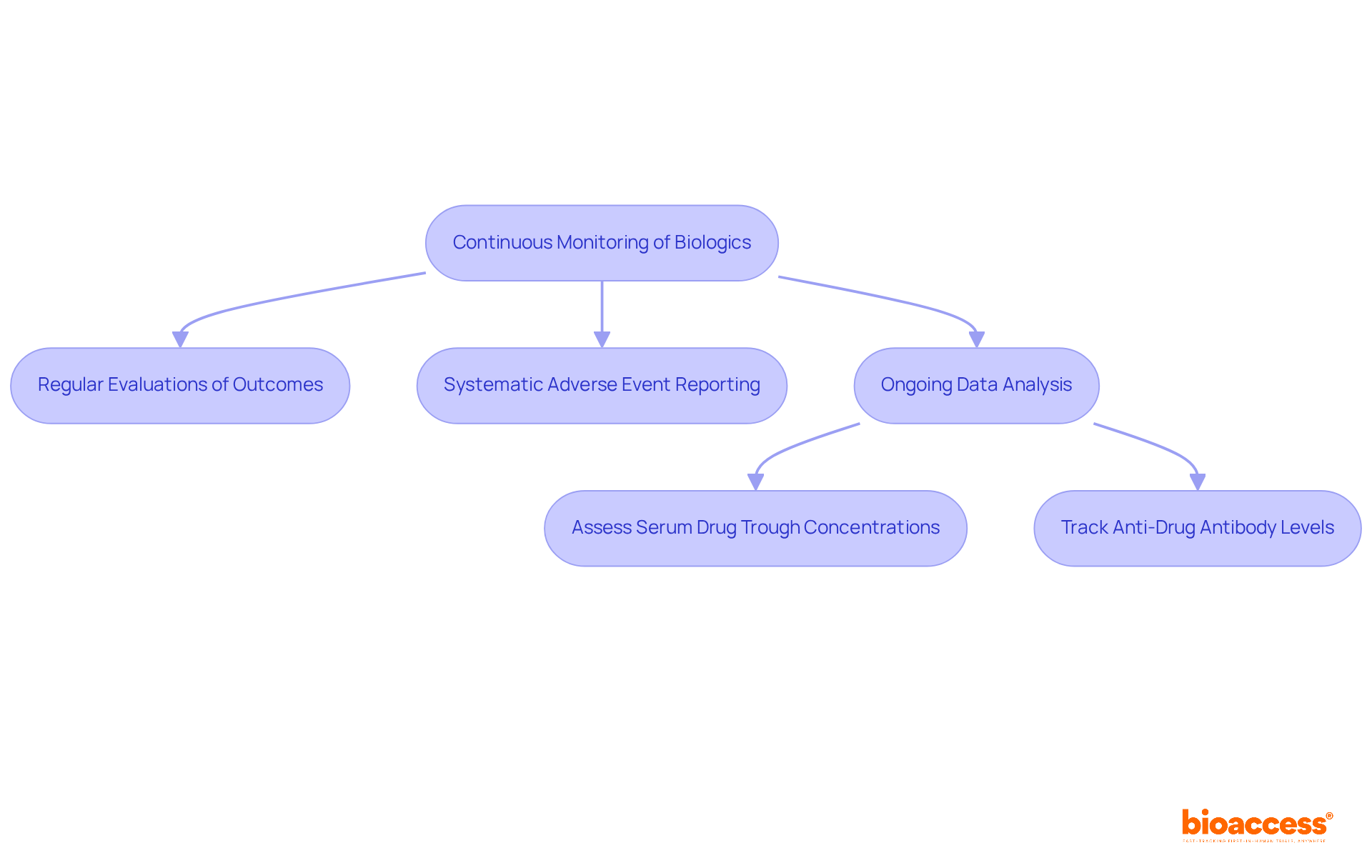
Ongoing assessment is crucial for the post-approval monitoring of biologics in Australia to ensure their long-term security and effectiveness. This process includes:
For instance, guidelines highlight the significance of assessing serum drug trough concentrations and anti-drug antibody levels for individuals receiving infliximab (IFX) and adalimumab (ADA). Research has demonstrated that tracking serum biologic levels in individuals with inflammatory bowel disease (IBD) relates to clinical response rates and remission outcomes, underscoring the importance of therapeutic drug monitoring (TDM).
Significantly, as many as 30% of individuals with IBD may not respond to initial therapy, emphasizing the essential requirement for ongoing observation. By adopting a vigilant approach to post-approval monitoring of biologics in Australia, stakeholders can ensure that these products deliver therapeutic benefits while effectively minimizing risks to individuals. This proactive strategy not only enhances safety for individuals but also fosters public confidence in biologic therapies, ultimately contributing to improved health outcomes.
As Francis Peabody aptly stated, true healing involves genuinely caring for the patient’s well-being, which is paramount in the context of biologic therapies.
Post-approval monitoring of biologics in Australia stands as a pivotal element in safeguarding the safety and efficacy of these groundbreaking therapies. As underscored throughout this article, organizations like bioaccess® are at the forefront of refining monitoring processes, highlighting the critical nature of compliance, risk management, and collaboration among stakeholders. This comprehensive approach not only protects public health but also cultivates trust in biologic treatments.
Key insights reveal that robust monitoring frameworks - encompassing thorough risk management plans and proactive biovigilance systems - are indispensable for detecting potential safety signals and ensuring ongoing patient safety. The integration of patient feedback, technological advancements, and regulatory compliance further fortifies the monitoring landscape, enabling a more responsive and effective oversight process. Continuous engagement among healthcare providers, regulatory agencies, and patients is essential for overcoming existing challenges and adapting to the dynamic Medtech environment.
Looking ahead, the future of post-approval monitoring in Australia will be influenced by technological advancements and a steadfast commitment to patient-centered care. Stakeholders must prioritize collaboration and innovation to enhance monitoring practices, ensuring that biologics consistently meet the highest standards of safety and effectiveness. By embracing these changes, the healthcare community can significantly improve patient outcomes and sustain public confidence in biologic therapies.
What is bioaccess® and its role in post-approval monitoring of biologics in Australia?
bioaccess® is a leader in post-approval monitoring of biologics in Australia, leveraging its clinical research expertise to maintain compliance and quality standards. It facilitates quicker identification of alert signals and efficacy information, ensuring that monitoring meets health standards.
Why is post-approval monitoring important for biologics in Australia?
Post-approval monitoring is crucial for maintaining public trust and ensuring the safety of individuals. It helps identify potential risks and ensures that biologics remain safe and effective throughout their lifecycle.
What strategies does bioaccess® employ for post-approval monitoring?
bioaccess® employs comprehensive risk management plans and proactive biovigilance systems to protect patient health and support the ongoing development of innovative biologics in Australia.
What regulatory body oversees biologics monitoring in Australia?
The Therapeutic Goods Administration (TGA) is the primary regulatory body overseeing biologics monitoring in Australia.
What are the key guidelines established by the TGA for post-approval monitoring?
The TGA has established guidelines that emphasize ongoing safety assessments, robust risk management plans, and the systematic collection of real-world evidence to ensure the safety and efficacy of biologics.
What recent innovation has the TGA introduced to enhance the monitoring process?
In 2025, the TGA introduced rolling submissions for priority medicines, allowing for timely data submission as it becomes available, which significantly enhances the monitoring process.
What are the reporting requirements for manufacturers regarding adverse incidents?
Manufacturers are required to disclose any adverse incidents they encounter to ensure critical information is communicated and addressed swiftly.
What types of data are collected during post-approval monitoring of biologics?
The data collected includes adverse event reports, patient outcomes, and long-term effectiveness studies, which are essential for evaluating the ongoing safety profile of biologics.
What recent findings have been reported regarding adverse effects of biologics?
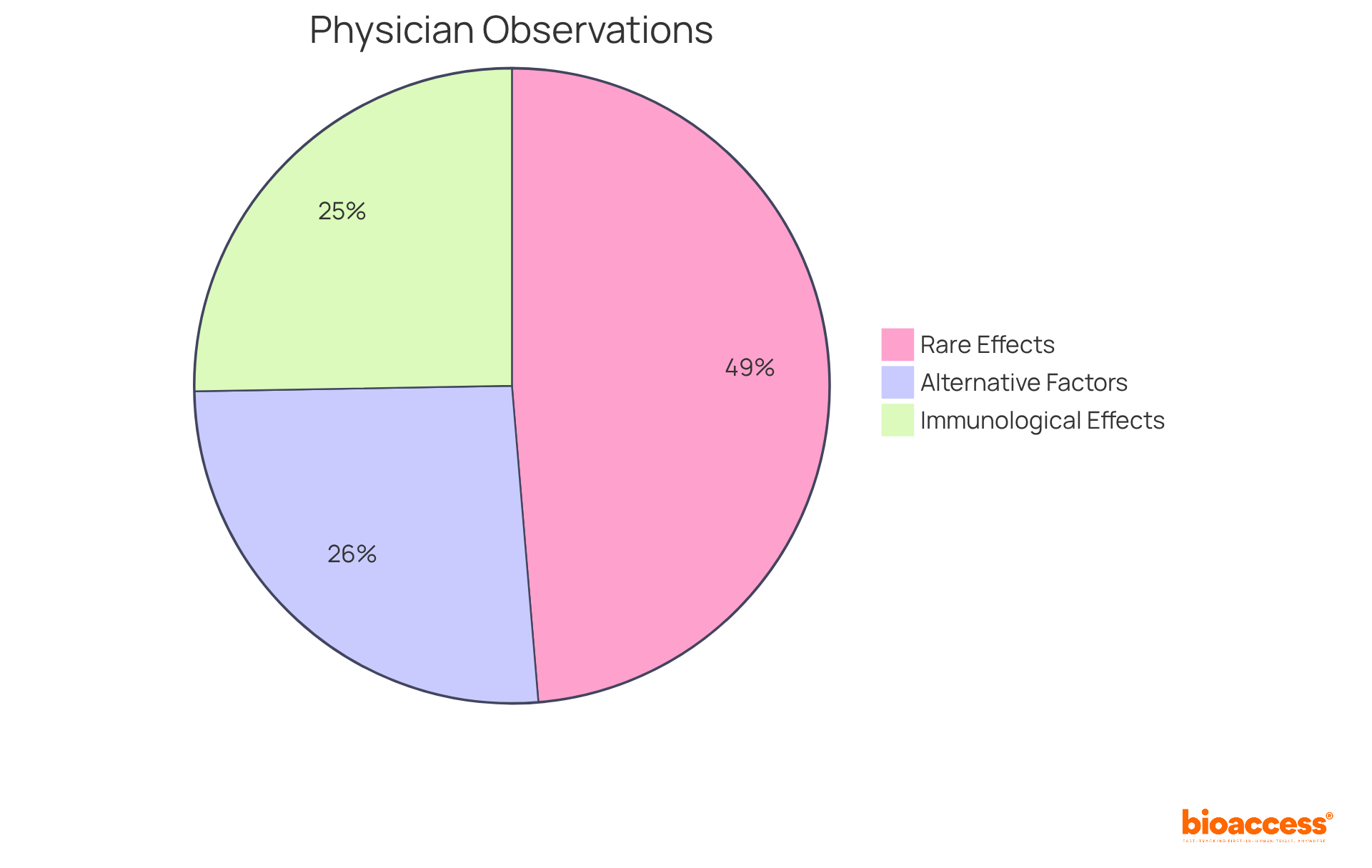
Recent data shows that 38.81% of physicians have observed immunological adverse effects, with 74.73% considering these effects to be rare. Additionally, 40% of doctors noted alternative factors unrelated to medication that could trigger adverse effects.
How can real-world evidence contribute to post-approval monitoring?
Incorporating real-world evidence, including outcomes data, enhances understanding of the long-term effects of biologics and aids in developing better management and treatment strategies for patient care.
Perception and Opinions of Physicians on the Safety and Tolerability of Biotherapeutics: A Survey Study
| European Journal of Medical and Health Sciences (https://ej-med.org/index.php/ejmed/article/view/2237)
Database of Adverse Event Notifications (DAEN) (https://tga.gov.au/safety/adverse-events/database-adverse-event-notifications-daen)
The whole experience of public hospital physicians from several specialties with biopharmaceutical effectiveness, safety, adverse drug reactions and interchangeability: A qualitative study (https://sciencedirect.com/science/article/pii/S2667276622000610)
Long-Term Real-World Post-approval Safety Data of Multiple Biosimilars from One Marketing-Authorization Holder After More than 18 Years Since Their First Biosimilar Launch - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC10684613)
Postapproval studies of drugs initially approved by the FDA on the basis of limited evidence: systematic review (https://bmj.com/content/357/bmj.j1680)