


The article delineates the essential obligations that distributors of medical devices must uphold in Latin America, underscoring the critical nature of compliance with local regulations for successful market entry and patient safety. It articulates various responsibilities, including:
These elements are vital for mitigating risks and ensuring effective distribution in a rapidly evolving healthcare market.
Navigating the intricate landscape of medical device distribution in Latin America presents unique challenges for distributors. The region's healthcare equipment market is projected to grow significantly, making it imperative for stakeholders to understand core obligations and legal requirements. This understanding is not merely a regulatory necessity; it serves as a strategic advantage that can unlock new opportunities and enhance operational efficiency. However, with evolving compliance standards and the increasing complexity of regulations, distributors must consider:
Bioaccess plays a crucial role in navigating the complex Latin America distributor obligations medical devices that healthcare product distributors face. With the healthcare equipment market in the region estimated at approximately $11 billion and poised for significant growth, understanding regulations is essential for effective market entry.
By leveraging extensive knowledge of oversight procedures and local market dynamics, Bioaccess equips distributors to meet Latin America distributor obligations medical devices efficiently. This support not only boosts operational efficiency but also upholds the integrity of healthcare product distribution, thereby ensuring patient safety.
As the framework for oversight evolves, particularly with anticipated changes in compliance requirements, the importance of adhering to the Latin America distributor obligations medical devices regulations cannot be overstated. Industry leaders assert that successfully navigating local regulations is vital for thriving in this competitive market, making Bioaccess's guidance indispensable for distributors seeking to establish a strong presence in Latin America.
Distributors in Latin America must understand their obligations related to medical devices amidst a complex landscape of legal requirements that vary significantly from country to country. Key regulations encompass:
For example, Brazil's regulatory framework requires that Class III and IV devices undergo a comprehensive approval process through ANVISA, while Class I and II devices can be registered via notification. Non-compliance with the Latin America distributor obligations for medical devices regulations can lead to severe penalties, including fines that may reach up to BRL 1 million and product recalls, which have notably increased in recent years.
In 2024, Brazil represented 1.1% of the global healthcare equipment clinical trials market, highlighting the critical importance of compliance for distributors seeking to thrive in this expanding sector. Compliance with Latin America distributor obligations for medical devices not only reduces risks but also unlocks significant market opportunities as the demand for healthcare products continues to escalate. Furthermore, the introduction of regulations such as RDC 751/2022 has streamlined the classification and approval processes, aligning them more closely with international standards and enhancing transparency.
Experts emphasize the necessity of comprehensive documentation and a strategic approach to effectively navigate compliance pathways related to Latin America distributor obligations for medical devices. Engaging local regulatory experts can significantly alleviate the challenges stemming from complex documentation requirements and communication barriers with authorities like ANVISA, INMETRO, and ANATEL. bioaccess offers extensive services, including:
All essential for successful compliance. Adhering to these regulations not only facilitates smoother operations but also builds trust among healthcare providers and patients, ultimately advancing public health. Additionally, foreign manufacturers must appoint a Brazilian Registration Holder to effectively navigate local regulations, which emphasizes the Latin America distributor obligations for medical devices and the necessity of understanding these legal requirements.
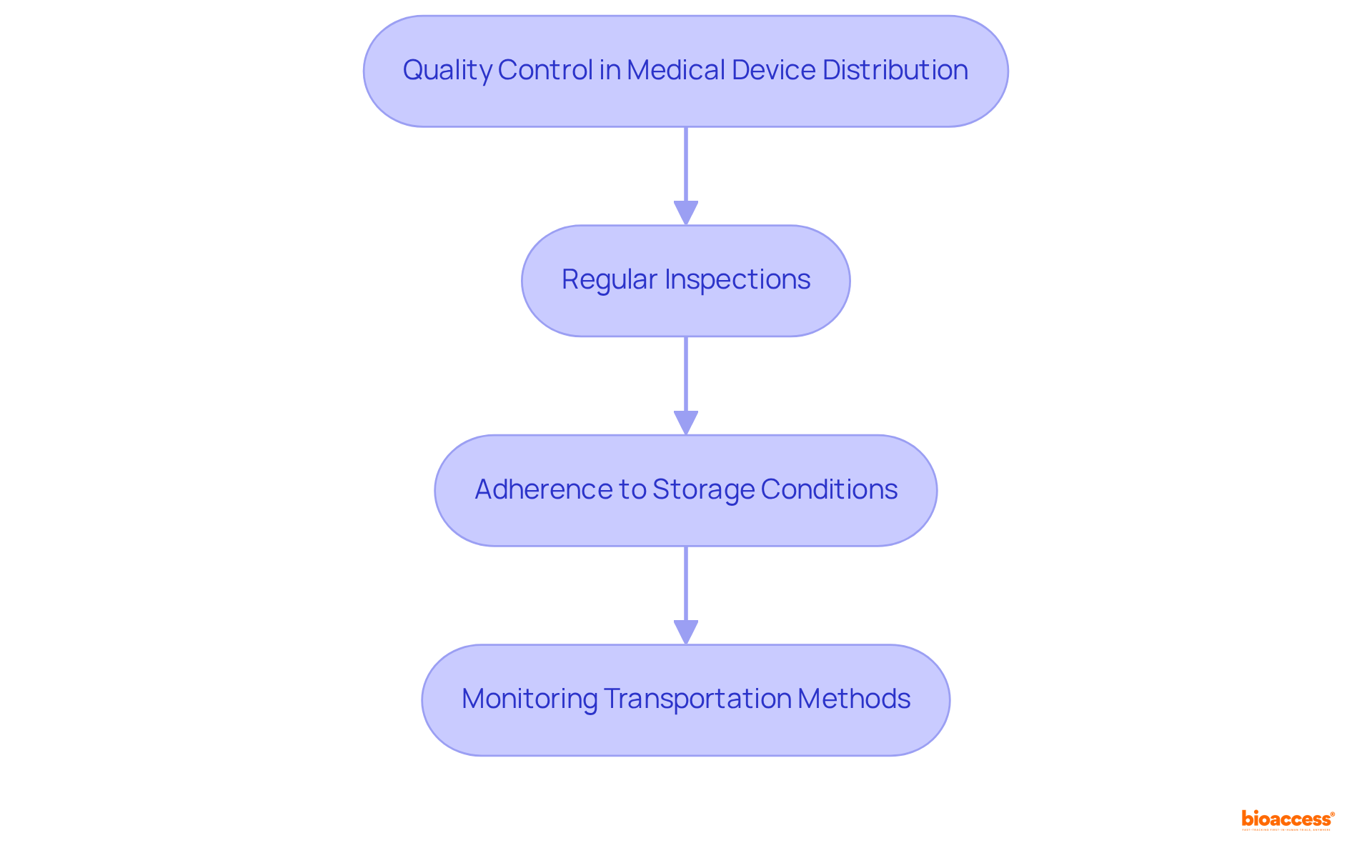
Implementing robust quality control measures is essential for the proper storage, handling, and transportation of health products, in compliance with the regulatory standards set forth by INVIMA, the Colombia National Food and Drug Surveillance Institute, as well as the Latin America distributor obligations for medical devices. This process necessitates:
Effective quality control protocols not only mitigate risks associated with failures but also significantly enhance patient safety. Notably, a study revealed that 85.1% of drug recalls stemmed from quality issues, underscoring the critical need for stringent quality oversight across both medications and the Latin America distributor obligations for medical devices. Furthermore, embracing a Quality Management System (QMS) aligned with the ISO 13485:2016 standard can streamline these processes, ensuring that all products meet the requisite safety and efficacy standards mandated by INVIMA. By prioritizing quality control, the obligations of Latin America distributors of medical devices can cultivate trust with healthcare providers and patients alike, ultimately contributing to improved health outcomes.
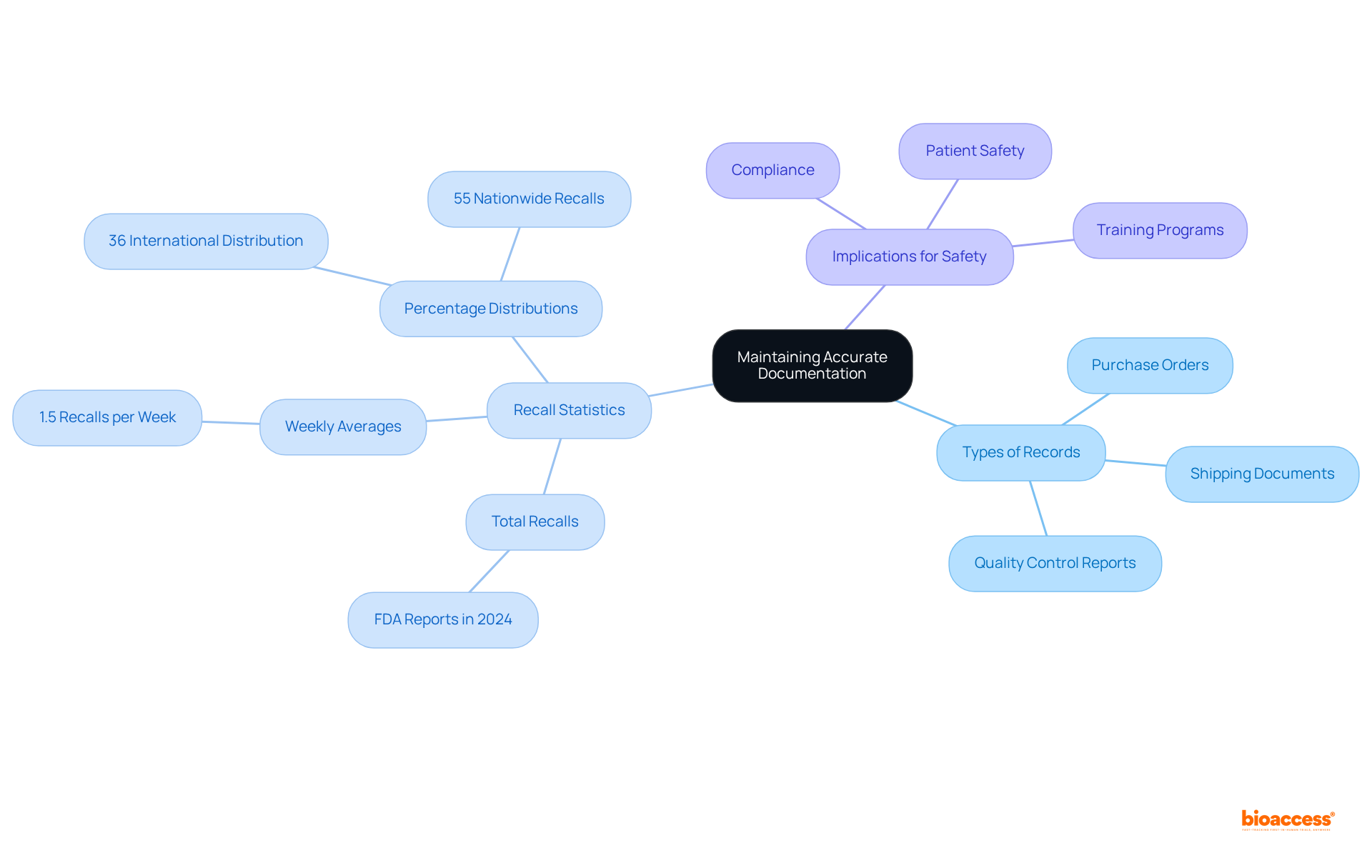
Distributors are mandated to maintain meticulous records of all transactions, including purchase orders, shipping documents, and quality control reports. This documentation is critical for demonstrating compliance during inspections and audits, as it provides a clear trail of accountability. Accurate records not only facilitate tracing products back to their source but are also vital in the event of a recall or safety issue.
In 2024, the FDA issued a total of 625 drug product recall enforcement reports, underscoring the necessity for robust documentation practices. Furthermore, the FDA announces an average of 1.5 product recalls each week, emphasizing the continual necessity for vigilance in record-keeping. Statistics reveal that 36% of recalls pertain to products with international distribution, while 55% of healthcare product recalls are nationwide, emphasizing the Latin America distributor obligations medical devices to uphold rigorous record-keeping standards.
Compliance officers assert that accurate documentation is not merely a regulatory requirement but a cornerstone of patient safety and operational efficiency. As the healthcare equipment landscape evolves in 2025, characterized by increasing complexity and a rise in recalls, the significance of maintaining accurate records cannot be overstated. To enhance adherence and safety, hospitals should invest in training programs for staff on documentation requirements and best practices.
Establishing robust training programs for distributor personnel is essential for meeting the Latin America distributor obligations for medical devices in the healthcare equipment sector. These programs must address critical areas, including:
Regular training sessions not only reinforce adherence standards but also keep staff informed about updates or changes in regulations. As medical devices grow increasingly complex, the necessity for effective training strategies becomes paramount. Organizations are progressively adopting interactive training techniques to enhance engagement and retention, which is vital for fostering a culture of adherence.
Moreover, the integration of emerging technologies, such as virtual reality and e-learning platforms, is revolutionizing training delivery, rendering it more accessible and effective. By prioritizing comprehensive training, distributors can significantly mitigate risks associated with non-compliance to Latin America distributor obligations for medical devices while enhancing their operational efficiency.
Distributors must prioritize active interaction with oversight agencies, such as INVIMA, to cultivate strong relationships that significantly enhance adherence efforts. INVIMA, the Colombia National Food and Drug Surveillance Institute, is tasked with inspecting and supervising the marketing and manufacturing of health products, including medical devices. This responsibility encompasses:
Such proactive involvement allows distributors to stay informed about legal changes regarding Latin America distributor obligations for medical devices and adjust their compliance strategies accordingly. For instance, the Costa Rican Drug Institute conducted 26 inspections in 2024, while DIGEMID completed 99 GPP certification inspections from August 2022 to August 2024. These figures underscore the importance of understanding local governance landscapes.
Furthermore, effective engagement strategies foster enhanced trust and cooperation, which are vital for navigating the complexities of device distribution in the region. Industry leaders emphasize that nurturing these connections not only aids in adherence to Latin America distributor obligations for medical devices but also empowers distributors to respond more effectively to evolving oversight demands, ultimately improving patient safety and access to innovative healthcare solutions. Transparency and trust between the pharmaceutical industry and regulatory authorities, such as INVIMA, are crucial, as affirmed by experts in the field.
Distributors have critical Latin America distributor obligations regarding post-market surveillance to evaluate the performance of medical devices in the field. This essential process involves:
By engaging actively in post-market monitoring, distributors can ensure ongoing compliance and significantly enhance the overall safety of healthcare products. In Colombia, this crucial process is overseen by INVIMA, the National Food and Drug Surveillance Institute, which plays an integral role in the regulation of healthcare instruments. As a Level 4 health authority recognized by PAHO/WHO, INVIMA ensures that distributors comply with Latin America distributor obligations for medical devices, thereby bolstering the safety and efficacy of healthcare products available in the market.
Furthermore, distributors are required to collaborate with clinical trial management services, which include:
to guarantee that all performance aspects are monitored effectively and reported accurately.
Distributors in the healthcare equipment industry must prioritize staying informed about compliance changes and market trends that could significantly impact their operations. Engaging with industry publications, attending conferences, and participating in professional organizations are effective strategies for knowledge acquisition.
For instance, the Latin America healthcare equipment compliance market is projected to grow from USD 260.7 million in 2024 to USD 446.2 million by 2030, reflecting a compound annual growth rate (CAGR) of 9.4%. This growth underscores the necessity for distributors, particularly regarding [Latin America distributor obligations](https://marketdataforecast.com/market-reports/latin-america -implantable-medical-devices-market) for medical devices, to adapt their strategies in response to evolving regulatory requirements and market demands.
Furthermore, with the increasing prevalence of chronic illnesses—which are expected to account for 73% of all fatalities worldwide by 2030—the demand for innovative health technologies is rising. By proactively acquiring knowledge, distributors can better position themselves to navigate these changes and capitalize on emerging opportunities in the market.
Distributors must fulfill their Latin America distributor obligations regarding medical devices by adopting robust risk management strategies to effectively identify and mitigate potential risks in medical device distribution. This involves:
By proactively addressing these risks, Latin America distributor obligations for medical devices not only enhance operational resilience but also ensure compliance with strict legal standards, particularly those outlined in ISO 14971:2019, which emphasizes the significance of ongoing risk management and post-market surveillance obligations.
As Lee Chickering, a Client Operations Manager in the life sciences industry, emphasizes, a proactive approach to managing potential hazards is crucial for ensuring patient safety and maintaining trust in the healthcare system. Successful risk assessment practices include:
Notably, McKinsey estimates that non-routine quality events drain the industry between US $2.5-5 billion annually, underscoring the financial imperative for robust risk management strategies. Distributors should also consider applying practical suggestions such as:
Distributors play a pivotal role in upholding high ethical standards within their operations, especially regarding comprehensive clinical trial management services. This commitment involves ensuring transparency in all transactions, actively avoiding conflicts of interest, and prioritizing patient safety above all else. By fostering an ethical culture, distributors not only build trust with stakeholders but also enhance the overall integrity of the medical device industry.
The services offered by bioaccess include:
These services are crucial in ensuring that these ethical standards are upheld. Notably, consistent branding, which can increase revenue by up to 23%, is closely linked to ethical operations, reinforcing the idea that integrity is essential for long-term success. In this landscape, transparency transcends mere regulatory compliance; it serves as a fundamental driver of brand trust and loyalty among healthcare professionals and patients alike.
Navigating the complexities of distributor obligations for medical devices in Latin America is essential for ensuring compliance and operational success in this rapidly evolving market. The key responsibilities outlined highlight the critical need for distributors to understand and adhere to various regulations, from registration and quality control to documentation and ethical standards. By prioritizing these obligations, distributors can not only mitigate risks but also enhance patient safety and trust in healthcare systems.
The importance of maintaining accurate records, implementing comprehensive training programs, and building strong relationships with regulatory authorities cannot be overstated. Each of these elements plays a crucial role in fostering compliance and operational efficiency, ultimately contributing to the overall integrity of medical device distribution in Latin America. Furthermore, engaging in post-market surveillance and staying informed about regulatory changes are indispensable strategies for distributors aiming to thrive in this competitive landscape.
As the demand for healthcare products continues to rise, it is imperative for distributors to adopt a proactive approach in fulfilling their obligations. By investing in training, risk management, and ethical practices, distributors can position themselves as trusted partners in the healthcare ecosystem. Embracing these responsibilities not only supports compliance but also paves the way for innovation and improved health outcomes across the region.
What is the role of Bioaccess in the distribution of medical devices in Latin America?
Bioaccess helps distributors navigate the complex obligations related to medical devices in Latin America, ensuring compliance with regulations and boosting operational efficiency while maintaining patient safety.
What are the key regulations that medical device distributors in Latin America must understand?
Distributors must understand regulations related to the registration of medical equipment, adherence to labeling standards, and compliance with import/export laws specific to each country.
What are the consequences of non-compliance with medical device regulations in Latin America?
Non-compliance can lead to severe penalties, including fines up to BRL 1 million and product recalls, which have become more common in recent years.
How does Brazil's regulatory framework affect medical device approval?
Brazil requires that Class III and IV medical devices undergo a comprehensive approval process through ANVISA, while Class I and II devices can be registered via notification.
What is the significance of compliance with Latin America distributor obligations for medical devices?
Compliance reduces risks and unlocks significant market opportunities as demand for healthcare products continues to grow, particularly in Brazil, which is expanding its role in the global healthcare equipment market.
What services does Bioaccess offer to assist with compliance?
Bioaccess offers services including feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting to help distributors comply with regulations.
What quality control measures are essential for medical device distribution?
Essential measures include regular inspections, adherence to specified storage conditions, and vigilant monitoring of transportation methods to ensure compliance with regulatory standards.
How can quality control impact patient safety in medical device distribution?
Effective quality control protocols mitigate risks associated with product failures and enhance patient safety, as a significant percentage of drug recalls are linked to quality issues.
What is the importance of adopting a Quality Management System (QMS) for distributors?
Implementing a QMS aligned with ISO 13485:2016 standards streamlines processes, ensuring that all products meet safety and efficacy standards required by regulatory authorities.
What is required for foreign manufacturers to navigate local regulations in Brazil?
Foreign manufacturers must appoint a Brazilian Registration Holder to effectively navigate the local regulations related to medical devices.