


The article titled "7 Local Representative Service Mexico Quotes for Clinical Research" underscores the diverse services available in Mexico that bolster clinical research endeavors. It emphasizes the benefits of local expertise, swift regulatory processes, and customized insurance and logistics solutions, all of which significantly enhance the efficiency and success of clinical trials in the region.
The landscape of clinical research in Mexico is rapidly evolving, propelled by an increasing demand for innovative solutions and local expertise. This market is projected to grow significantly, presenting stakeholders with a unique opportunity to leverage local representative services that enhance efficiency and compliance in research operations.
However, navigating the complexities of this dynamic environment raises critical questions about how to effectively harness these resources while addressing potential challenges. As we delve deeper into this topic, it becomes essential to explore the Medtech landscape and the pivotal role of bioaccess in overcoming key obstacles faced in clinical research.
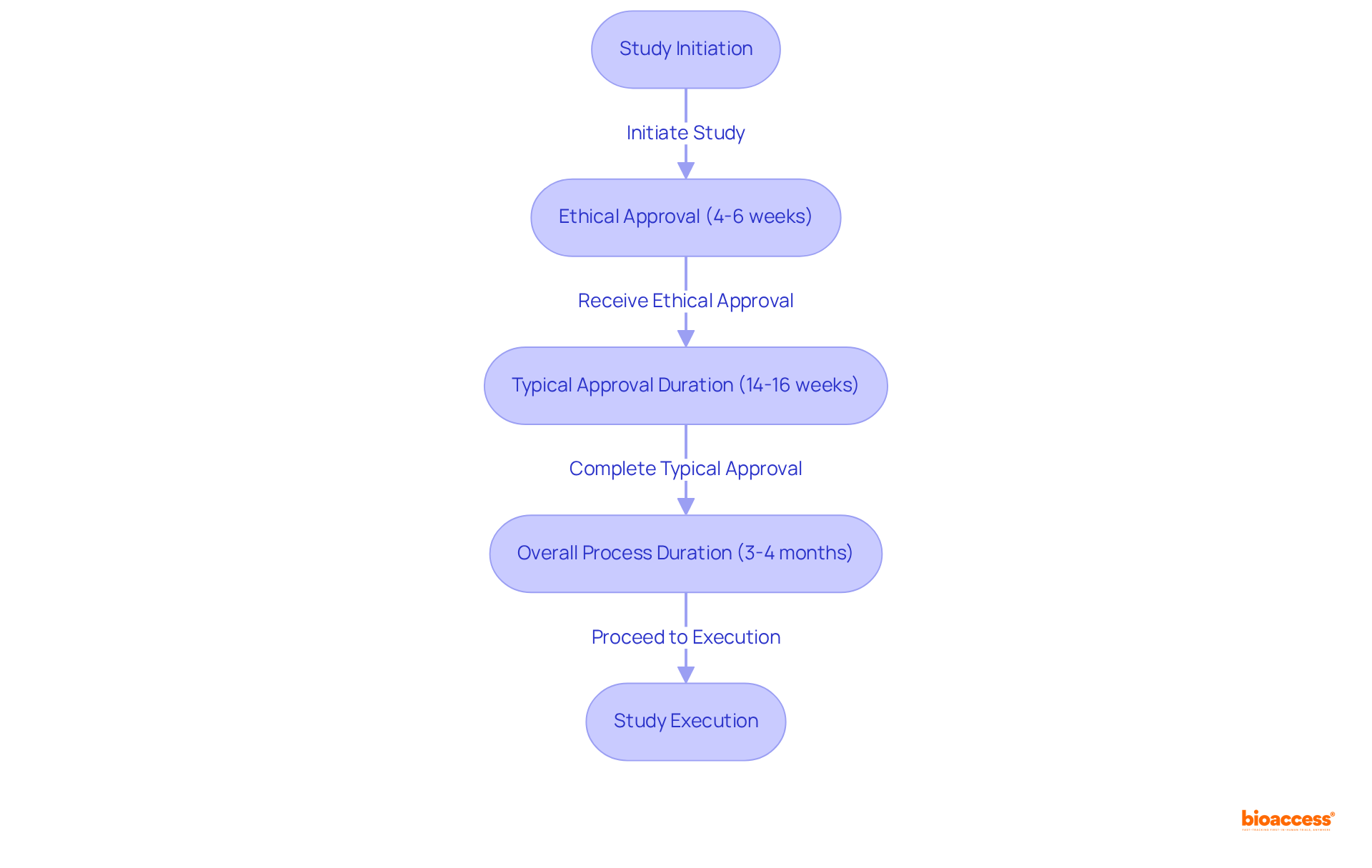
bioaccess® leverages over 15 years of experience in Latin America to deliver exceptional research services for Medtech, Biopharma, and Radiopharma innovators. With ethical approvals secured in an impressive 4-6 weeks, bioaccess® enables a swift start to studies, fully capitalizing on Mexico's advantageous regulatory environment.
Typically, the approval duration for research studies in Mexico ranges from 14 to 16 weeks, while the overall approval and setup process generally spans 3-4 months, positioning it competitively with other Latin American countries. Moreover, the organization’s local expertise significantly enhances patient recruitment and study execution, ensuring high retention rates and fostering trust in the physician-patient relationship.
This combination of rapid regulatory processes and extensive local knowledge establishes bioaccess® as a preferred partner for research in Mexico, a market projected to reach USD 510.4 million by 2030, growing at a CAGR of 6.9% from 2024 to 2030.
Nonetheless, it is crucial to acknowledge the challenges within the regulatory landscape, including complexities that may lead to potential delays.
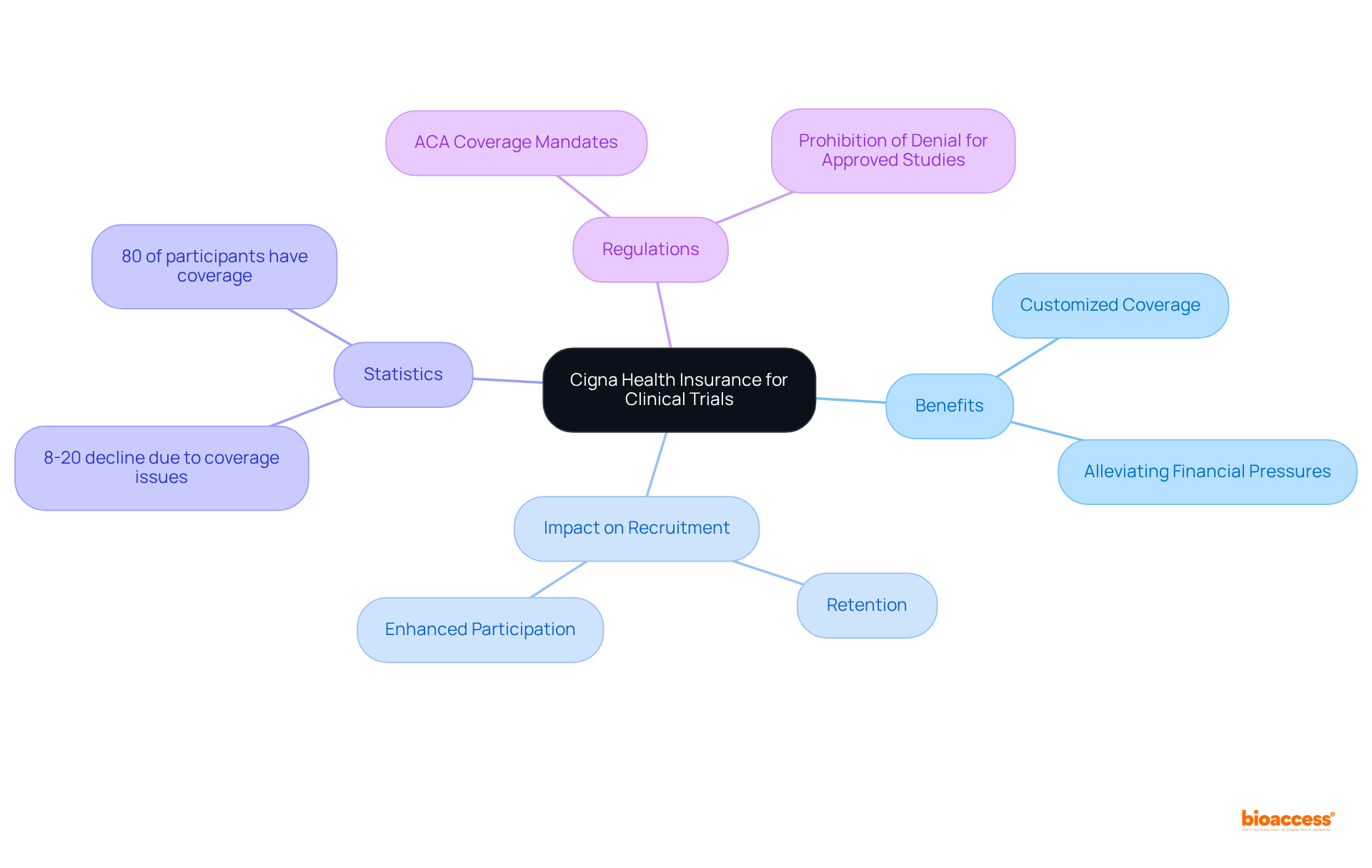
Cigna offers customized health coverage plans specifically designed for research participants, encompassing standard care alongside any additional medical costs incurred during the study. This extensive coverage significantly alleviates financial pressures, thereby enhancing participant recruitment and retention—two pivotal elements for the success of research studies.
Research indicates that health coverage plays a critical role in trial participation, with numerous patients citing coverage issues as a barrier to enrollment. In fact, studies reveal that 8 to 20% of eligible patients decline participation due to potential denial of coverage. Furthermore, 80% of cancer research study participants possess health coverage that contributes to at least part of their care, underscoring the overall positive impact of coverage on participation rates.
By addressing these concerns, Cigna not only streamlines recruitment processes but also cultivates a supportive environment that encourages sustained participation, ultimately advancing the progress of medical studies. According to the ACA, health providers are prohibited from denying coverage for involvement in an authorized study for cancer or another life-threatening illness, highlighting the crucial significance of coverage in medical investigations.
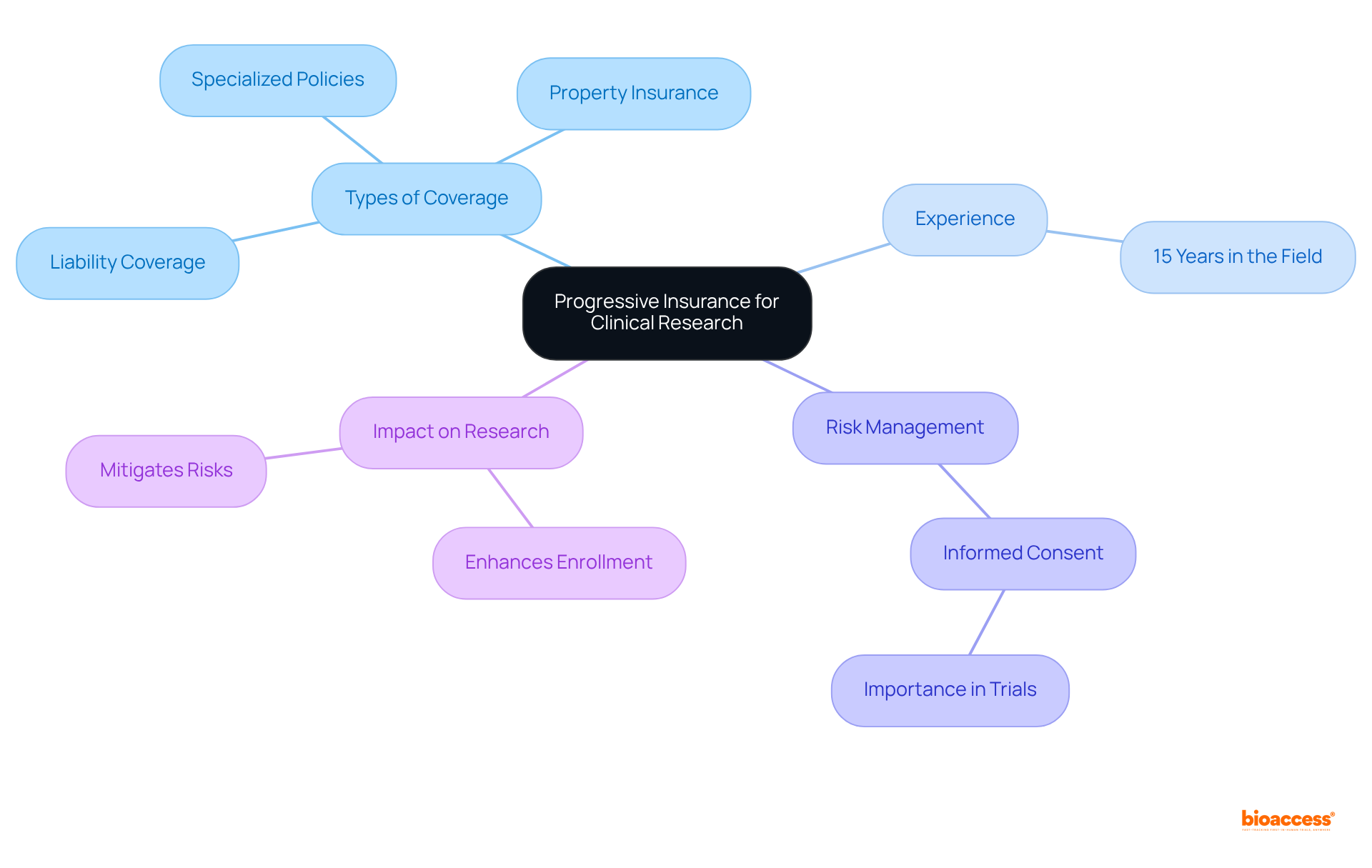
Progressive offers tailored coverage options designed to address the distinct hazards associated with medical trial activities. Their extensive offerings include:
With over 15 years of experience in the field, Progressive understands the intricacies of medical studies and provides customized coverage that not only protects against potential liabilities but also fosters trust among participants, ultimately enhancing enrollment in vital medical research. This approach is particularly crucial as the landscape of clinical studies evolves, introducing new risks, such as those linked to decentralized studies and emerging technologies, a reality underscored during the COVID-19 pandemic.
Furthermore, informed consent remains a critical component in ensuring that participants understand their involvement in studies, an aspect Progressive emphasizes in their risk management strategies. By effectively mitigating these risks, Progressive bolsters the viability of experimental operations, facilitating smoother project execution and addressing essential elements like medical expenses and rehabilitation costs that may arise during trials.
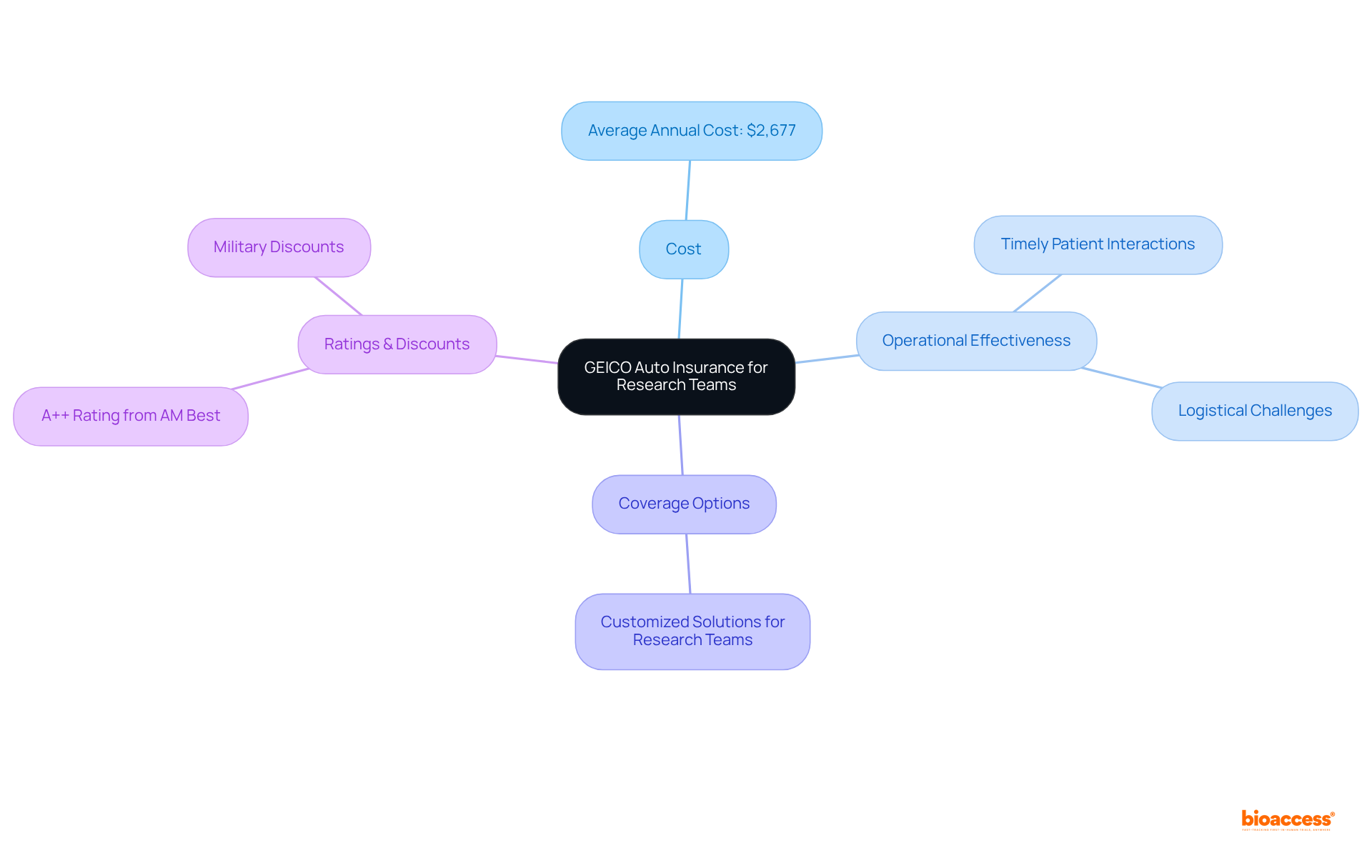
Dependable vehicle coverage is imperative for research logistics, particularly in 2025, as it directly influences operational effectiveness. GEICO offers customized auto coverage solutions specifically designed for research teams, ensuring that team members can travel to various study locations without the burden of unexpected vehicle-related costs.
With the average annual cost of full coverage car insurance being $2,677, having reliable insurance is essential for effectively managing operational expenses. This coverage not only provides competitive rates but also extensive options that address the specific logistical challenges faced during research trials.
Experts emphasize that reliable transportation is vital for maintaining timely patient interactions and facilitating seamless operations. Furthermore, GEICO's A++ rating from AM Best and its competitive military discounts further establish it as a valuable partner in the healthcare sector.
The Nature Conservancy provides crucial insights into environmental well-being that can significantly influence medical study methodologies. Understanding the impact of environmental factors on health outcomes—accounting for approximately 14% of the total burden of disease in the UK, as reported by the World Health Organization—enables researchers to develop more effective studies and enhance patient care.
Collaborating with organizations like the Nature Conservancy allows research to address broader health determinants, thereby amplifying the overall significance of findings. Dr. Margaret Chan emphasizes that a healthy planet is essential for human well-being, underscoring the necessity of integrating environmental considerations into medical research.
Additionally, the repercussions of climate change on mental health conditions, including anxiety and depression, underscore the urgency of this approach. Effective partnerships, exemplified in various case studies, demonstrate the tangible benefits of incorporating environmental factors into research.
With the forthcoming 2025 Global Conference on Climate and Health, there lies a timely opportunity to delve deeper into these critical intersections.
Hapag-Lloyd excels in delivering logistics solutions tailored to the specific needs of medical studies. Their services are designed to ensure the secure and timely delivery of research materials, including sensitive biological specimens and investigational products. By leveraging Hapag-Lloyd's expertise, research teams can effectively minimize delays and comply with stringent regulatory requirements, thereby enhancing overall study efficiency.
As we look toward 2025, the logistics landscape is transforming, with an increasing focus on real-time tracking and automation to tackle transportation challenges. The global healthcare research logistics market is projected to generate a revenue of US$ 4.1 billion in 2024, with a compound annual growth rate (CAGR) of 9.3%, underscoring the growing demand for efficient logistics services.
The integration of advanced technologies is becoming increasingly essential for maintaining compliance and ensuring the integrity of research materials throughout the supply chain. Hapag-Lloyd's case studies illustrate successful implementations of these logistics solutions, showcasing their commitment to supporting research initiatives with precision and reliability.
Furthermore, addressing the challenges faced in transporting trial materials, such as regulatory compliance and the need for timely delivery, is critical for the success of clinical trials.
USAA delivers tailored financial and protection solutions specifically designed for medical researchers, addressing the critical need for efficient management of trial-related expenses. Their services encompass:
By collaborating with USAA, medical researchers can concentrate on advancing their studies while effectively managing financial risks. This strategic partnership not only enhances operational efficiency but also aligns with contemporary trends emphasizing the necessity for robust protection solutions within the research environment.
As bioaccess® states, "solid insurance is the only ethical way forward," underlining the ethical imperatives of financial management in research endeavors. Furthermore, with 55% of respondents indicating that at least one cost-related factor significantly impacts their participation in trials, the urgency of addressing these financial barriers becomes increasingly apparent. Bioaccess®'s focus on early-phase studies and their market access services further underscores the vital role of financial solutions in facilitating successful trials.
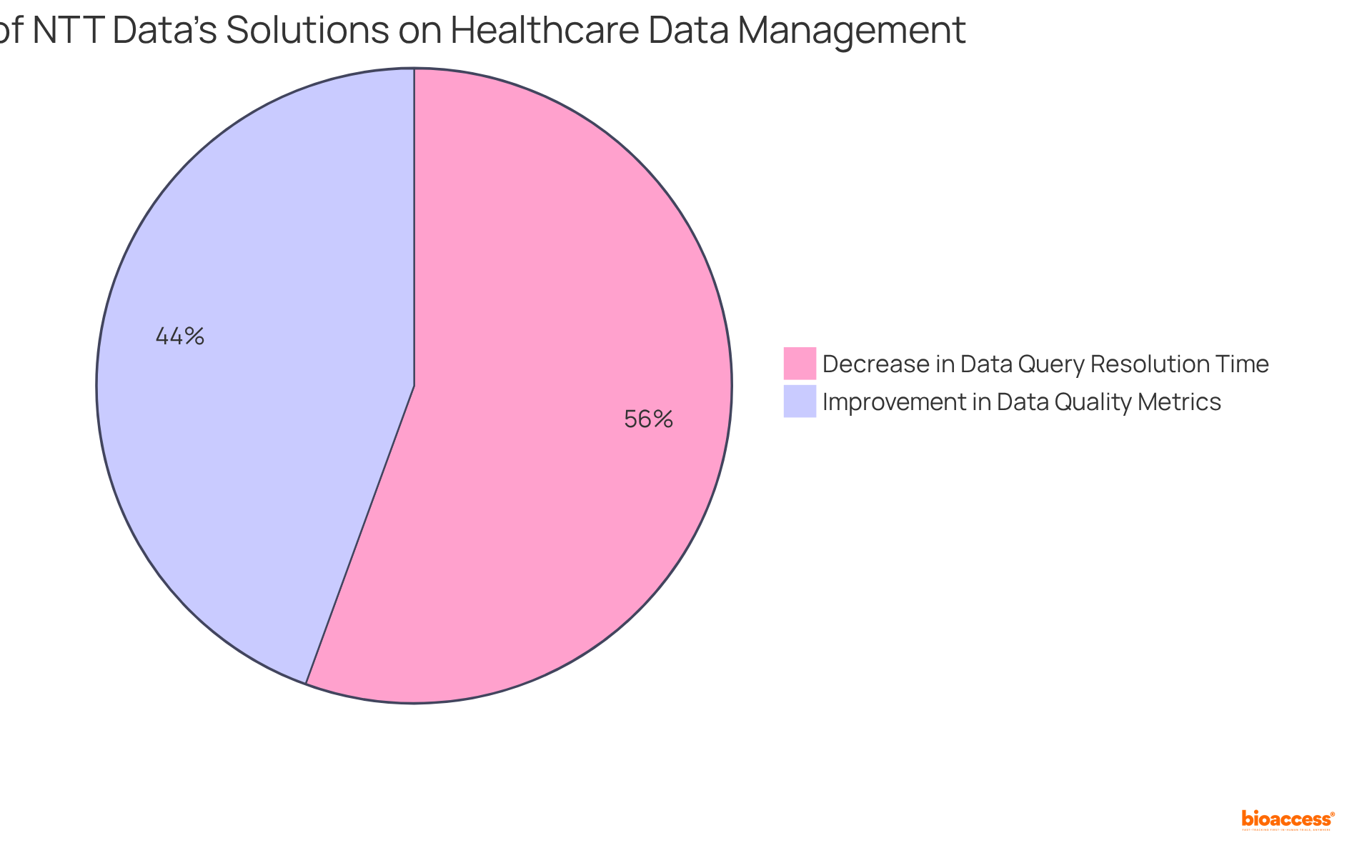
NTT Data stands at the forefront of transforming healthcare data management through innovative technology solutions. Their platforms streamline data collection, analysis, and reporting, enabling researchers to efficiently handle large datasets while adhering to stringent regulatory standards. By utilizing NTT Data's advanced solutions, researchers can significantly enhance data quality, achieving a 28% improvement in data quality metrics, and expedite the overall research timeline.
Moreover, studies utilizing AI-driven data management solutions indicate a 35% decrease in data query resolution time, underscoring the effectiveness of these technologies. As the data management environment evolves, particularly with the anticipated incorporation of AI and machine learning technologies by 2025—which is projected to reach $4.9 billion by 2028—NTT Data's contributions are essential for ensuring compliance and enhancing efficiency in medical studies.
As Sarah Lee observes, 'Data management in the healthcare field includes the gathering, purification, and oversight of information from trials and studies—a procedure that has changed significantly with technological progress.
Air Canada provides a comprehensive suite of travel services specifically designed for medical study teams operating in Mexico. Their offerings encompass flexible booking options, customized group travel arrangements, and dedicated support for managing travel logistics. By collaborating with Air Canada, research teams can streamline their travel processes, ensuring that all logistical needs are thoroughly addressed. This partnership allows researchers to focus on their primary objectives, thereby enhancing overall productivity and efficiency in their studies.
Given that the medical research supply and logistics market is projected to grow at a CAGR of 7.9% by 2032, the importance of effective travel logistics is paramount. As Mariam Faizullabhoy noted, 'Effective supply and logistics management are essential for ensuring the smooth and ethical execution of research studies.
Canada Post offers specialized secure shipping options tailored for research materials, ensuring compliance with stringent regulatory standards. Their services are meticulously designed to safeguard sensitive materials during transit, significantly mitigating the risk of damage or loss. By leveraging Canada Post's expertise, medical researchers can guarantee prompt and secure delivery of their materials, a critical factor in preserving the integrity of research studies.
Notably, the medical study supplies market is valued at $3.9 billion in 2023 and is projected to reach $6.3 billion by 2028, underscoring the necessity of secure shipping in this burgeoning sector. Furthermore, logistics expert Priyanka Bhendale highlights that IoT devices facilitate continuous monitoring of temperature and environmental conditions during transportation, thereby enhancing compliance and safety.
The integration of contemporary trends in secure shipping solutions, such as the collaboration between SkyCell and Marken for temperature-sensitive medications, illustrates practical applications that can further bolster the operational success of studies. To ensure compliance in shipping clinical trial materials, researchers should consider implementing robust tracking systems and engaging specialized logistics providers.
The landscape of clinical research in Mexico is significantly enhanced by a diverse array of local representative services that improve the efficiency and effectiveness of studies. By leveraging local expertise, organizations can adeptly navigate the complexities of regulatory approvals, participant recruitment, and logistical challenges, ultimately accelerating the research process. This article has highlighted key players such as bioaccess®, Cigna, and Hapag-Lloyd, showcasing how their specialized services cater to the unique needs of clinical trials.
Throughout this discussion, critical insights have emerged. The rapid approval times offered by bioaccess® and the comprehensive health coverage from Cigna exemplify how local representatives can mitigate barriers to participation and streamline processes. Furthermore, the tailored insurance solutions from Progressive and the logistics expertise of Hapag-Lloyd underscore the importance of addressing both operational risks and material transport needs. Each of these elements plays a pivotal role in ensuring that clinical trials are conducted smoothly and effectively.
As the demand for clinical research services continues to grow, particularly in emerging markets like Mexico, the significance of local partnerships cannot be overstated. Researchers and organizations are strongly encouraged to explore these local representative services to enhance their study designs and operational strategies. By doing so, they not only improve their chances of success but also contribute to the advancement of medical knowledge and patient care on a broader scale. Embracing local expertise is not merely beneficial; it is essential for the future of clinical research.
What services does bioaccess® provide for clinical research in Mexico?
bioaccess® offers research services for Medtech, Biopharma, and Radiopharma innovators, leveraging over 15 years of experience in Latin America.
How quickly can bioaccess® secure ethical approvals for studies?
bioaccess® can secure ethical approvals in an impressive 4-6 weeks.
What is the typical duration for research study approvals in Mexico?
The typical approval duration for research studies in Mexico ranges from 14 to 16 weeks.
What is the overall approval and setup process duration for clinical studies in Mexico?
The overall approval and setup process generally spans 3-4 months.
What advantages does bioaccess® offer in terms of patient recruitment and study execution?
bioaccess®'s local expertise enhances patient recruitment and study execution, ensuring high retention rates and fostering trust in the physician-patient relationship.
What is the projected market size for clinical research in Mexico by 2030?
The market for clinical research in Mexico is projected to reach USD 510.4 million by 2030, growing at a CAGR of 6.9% from 2024 to 2030.
What challenges exist within the regulatory landscape for clinical research in Mexico?
The regulatory landscape has complexities that may lead to potential delays in the research process.
What health coverage does Cigna provide for clinical trial participants?
Cigna offers customized health coverage plans that encompass standard care and any additional medical costs incurred during the study.
How does health coverage impact participant recruitment in clinical trials?
Health coverage significantly alleviates financial pressures, enhancing participant recruitment and retention, as many eligible patients decline participation due to coverage issues.
What percentage of eligible patients decline participation in trials due to potential denial of coverage?
Studies reveal that 8 to 20% of eligible patients decline participation due to potential denial of coverage.
What does the Affordable Care Act (ACA) state regarding health coverage for trial participants?
According to the ACA, health providers are prohibited from denying coverage for involvement in an authorized study for cancer or another life-threatening illness.
What insurance solutions does Progressive offer for clinical research operations?
Progressive offers tailored coverage options including liability coverage, property insurance, and specialized policies for study sponsors and contract organizations.
How does Progressive address the evolving risks associated with clinical studies?
Progressive provides customized coverage that protects against potential liabilities, especially in the context of decentralized studies and emerging technologies.
What role does informed consent play in clinical research according to Progressive?
Informed consent is emphasized as a critical component to ensure participants understand their involvement in studies, which is part of Progressive's risk management strategies.
How does Progressive facilitate smoother project execution in clinical trials?
By effectively mitigating risks and addressing essential elements like medical expenses and rehabilitation costs, Progressive bolsters the viability of experimental operations.