


This article delineates the essential steps for achieving GUDID compliance in medical device registration. It underscores the critical importance of understanding UDI requirements, registering with accredited issuers, and accurately entering data into the GUDID database. Such a comprehensive approach is bolstered by expert insights and practical examples, illustrating how adherence to these steps significantly enhances product traceability, regulatory compliance, and overall patient safety within the medical industry.
Navigating the intricate landscape of medical device registration presents significant challenges, particularly with the looming deadlines for GUDID compliance. As manufacturers endeavor to meet stringent regulatory standards, grasping the essential steps for compliance is paramount for success in a competitive market.
This article provides a comprehensive checklist that delineates the crucial actions manufacturers must undertake to achieve GUDID compliance. It explores the benefits of adherence, not only for securing regulatory approval but also for enhancing patient safety and operational efficiency.
How can manufacturers effectively streamline their compliance processes while remaining ahead of the evolving regulatory landscape?
bioaccess® excels in accelerating adherence for medical products by leveraging its extensive knowledge of regulatory frameworks across Latin America, the Balkans, and Australia. With an impressive capability to secure ethical approvals in just 4-6 weeks and enhance enrollment rates by 50%, bioaccess® offers a streamlined approach for manufacturers to navigate the complexities of registration. This tailored strategy not only ensures prompt adherence but also mitigates delays in the market entry of innovative medical devices.
Industry leaders underscore the critical importance of regulatory adherence, emphasizing its role in ensuring product safety and effectiveness in real-world settings. Successful registrations, such as those achieved by NuView for its liquid biopsy diagnostic test, exemplify the efficacy of bioaccess®'s strategies in facilitating timely market access.
As regulatory landscapes continue to evolve, the speed at which companies can achieve compliance will significantly influence their ability to capitalize on the burgeoning medical equipment market in Latin America, where over 10,000 medical products are already available globally.
Colombia emerges as a prime destination for clinical trials due to its cost efficiency, offering savings of over 30% compared to North America and Western Europe, alongside a regulatory review process that spans just 90-120 days. Furthermore, the Colombian government provides R&D tax incentives, including a 100% tax deduction for investments in science and technology, enhancing the appeal of conducting trials in the country.
The collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading hub for clinical trials in Latin America, supported by Colombia's Minister of Health. Additionally, the partnership with Welwaze Medical Inc. for the Celbrea® launch highlights bioaccess®'s commitment to facilitating market access and reinforces the necessity of partnering with organizations like bioaccess® to navigate regulatory challenges and expedite the commercialization of life-saving technologies.
The Unique Device Identifier (UDI) format comprises two essential elements: the Device Identifier (DI) and the Production Identifier (PI). The DI serves as a required, fixed element that identifies both the labeler and the specific version or model of the apparatus. In contrast, the PI provides supplementary details, including the lot or batch number and expiration date.
Mastering this format is vital for manufacturers, as it ensures precise input of equipment information into the GUDID. This accuracy is crucial for effective tracking and compliance, especially with the mandatory UDI/Device Registration period set to commence in Q4 2024, culminating in a compliance deadline by Q2 2026.
Regulatory specialists emphasize that adherence to UDI standards not only enhances traceability but also protects patients and clinicians by preventing counterfeit products from infiltrating the market. As producers prepare for these changes, understanding the GUDID format becomes a critical component of successful UDI registration and overall market readiness.
![]()
To satisfy UDI compliance standards, producers must enroll with an FDA-accredited UDI issuer, a crucial step for acquiring a unique UDI for each medical item. This UDI must be prominently displayed on the device label and packaging. The FDA recognizes several accredited issuing agencies, including:
Collaborating with an accredited issuer not only ensures that UDIs adhere to FDA regulations but also streamlines the process of entering the GUDID. Statistics indicate that manufacturers listed with UDI issuers achieve greater adherence rates, significantly lowering the risk of penalties and delays in market access. This proactive strategy is essential for producers aiming to navigate the complexities of regulatory adherence effectively. Experts like Ana Criado, Director of Regulatory Affairs and a consultant with extensive experience in biomedical engineering and health economics, emphasize the necessity of following these regulations to ensure successful market entry for medical products.

Obtaining GS1 standardized barcodes is essential for achieving gudid compliance. These barcodes must be displayed on labels and packaging in both human-readable and machine-readable formats. Currently, approximately 70% of medical instruments utilize GS1 barcodes for UDI regulations, highlighting the importance of gudid in the industry.
The GS1 system offers a globally recognized standard that enhances traceability, ensuring items are accurately identified throughout the supply chain. Notably, leading manufacturers have embraced GS1 barcodes to streamline their registration processes and improve inventory management.
Experts in supply chain management assert that effective barcode implementation not only facilitates regulatory compliance but also enhances patient safety by enabling the gudid for precise tracking of medical devices. Manufacturers must ensure their barcodes align with FDA requirements to mitigate potential issues during audits and inspections, thereby safeguarding their market position and reputation.

To sign up for an account, manufacturers must acquire a Data Universal Numbering System (DUNS) number, a distinct identifier issued by Dun & Bradstreet that confirms the legal identity of the labeler organization. This gudid number is crucial for guaranteeing the precision and traceability of the information provided to the database.
The average time to acquire a DUNS number can take up to 30 business days, making it essential for manufacturers to initiate this process early in their compliance journey to avoid potential delays. Industry leaders emphasize that efficient preparation, including obtaining a DUNS number, is vital for a successful submission. Adam Newman, Head of Marketing, asserts, "Effective preparation is essential for a successful submission."
Furthermore, producers who have effectively managed the registration process underscore the significance of the DUNS number and its relation to the gudid in simplifying their submissions and enhancing compliance. By prioritizing the acquisition of a DUNS number, which is free of charge, manufacturers can ensure they meet relevant requirements efficiently, thereby minimizing regulatory risks and facilitating smoother market access.

To ensure accurate categorization for submission to the GUDID, manufacturers must acquire a Global Medical Nomenclature (GMDN) code for their medical products. The GMDN provides a standardized terminology that enhances communication and understanding among all stakeholders involved. Each medical device is required to have at least one GMDN code, which aids regulatory bodies and healthcare providers in accurately identifying and categorizing devices. This classification is essential, as adherence to GMDN codes is increasingly mandated by regulatory authorities, including the GUDID, facilitating smoother market entry and alignment with international standards. As emphasized by specialists such as Ana Criado, Director of Regulatory Affairs, acquiring a GMDN code is crucial for compliance. Manufacturers can easily obtain GMDN codes through the GMDN Agency's website, ensuring they meet the necessary criteria for successful submissions.

The final phase in meeting the necessary standards necessitates the meticulous input of all required information into the database. Producers can choose to submit their data through the web application or via HL7 SPL XML file submissions, with the decision primarily influenced by their submission volume. For those submitting higher volumes, the HL7 SPL XML pathway presents significant advantages, enabling automated submissions that greatly streamline the process.
Precision and thoroughness are paramount; any discrepancies can result in regulatory issues and potential penalties. Industry statistics indicate that the typical adherence rate for producers entering information into the database hovers around 75%, highlighting the critical need for accuracy in submissions.
Post-submission, it is essential for manufacturers to consistently monitor their entries to maintain standards. This proactive approach not only mitigates risks but also ensures that all records correspond with device labeling and regulatory requirements, thereby enhancing the overall integrity of the submission process.
As regulatory experts emphasize, common pitfalls in GUDID submissions include:

Manufacturers face considerable challenges when navigating global Unique Device Identification (UDI) requirements, which differ significantly across countries. These challenges include diverse regulations, adherence timelines, and data submission procedures. For instance, the U.S. implemented UDI regulations in 2013, mandating adherence for Class III products by September 24, 2014, and Class I products by December 8, 2022. In contrast, Saudi Arabia has set deadlines for high-risk items by September 1, 2023, and medium to low-risk items by September 1, 2024. The European Union's UDI system is anticipated to be fully operational by the end of Q2 2025, with adherence beginning on January 1, 2026.
To effectively manage these complexities, manufacturers must prioritize staying informed about the latest regulatory updates and engage with local experts who possess a deep understanding of each market's nuances. Developing a comprehensive UDI strategy that accommodates these variations is essential. This strategy should incorporate automated data collection processes that capture critical attributes such as device identifiers and production identifiers, ensuring compliance with regulations like the FDA's requirements for gudid.
Moreover, leveraging platforms such as RegDesk, which provides regulatory insights across more than 120 markets, can facilitate the navigation of UDI regulations. This platform aids manufacturers in preparing and publishing international submissions, thereby streamlining regulatory efforts and minimizing the risk of penalties, which could lead to loss of business and reputation. By adopting these strategies and consistently reviewing their UDI approaches in response to evolving regulations, manufacturers can enhance their operational efficiency in the global marketplace.
Achieving UDI conformity presents significant advantages for both manufacturers and patients, particularly through the implementation of the gudid. For manufacturers, the gudid enhances product traceability, optimizes supply chain processes, and facilitates quicker recalls in the event of adverse incidents, which is essential for mitigating risks associated with equipment failures.
Experts, including Ana Criado, Director of Regulatory Affairs and a consultant with extensive experience in biomedical engineering and health economics, emphasize that adherence to gudid is critical for navigating the complex regulatory landscape in Colombia and beyond.
For patients, adherence to gudid guarantees accurate identification of medical instruments, which is vital for ensuring safety and improving the quality of care. Healthcare professionals assert that improved traceability through UDI systems, as facilitated by gudid, is directly linked to heightened patient safety, enabling timely alerts regarding recalls and compatibility issues.
Furthermore, a majority of moderate- and high-risk products are now labeled with a gudid, which underscores the pervasive compliance within the sector. By prioritizing gudid adherence, manufacturers not only meet regulatory requirements but also play a crucial role in enhancing patient outcomes and building trust in their products.
This commitment to safety and quality ultimately cultivates a more reliable healthcare environment, where patients can confidently rely on the tools that support their health. To fully leverage these benefits, manufacturers should actively participate in gudid training and stay informed about regulatory developments.

To achieve UDI compliance effectively, manufacturers must follow these essential steps:
Regulatory experts emphasize that understanding the intent behind UDI—primarily traceability—is crucial for successful implementation. As Jon Speer notes, "This should not be a foreign concept to anybody. This is a concept of traceability - that’s all a UDI is - traceability." By following these steps, manufacturers can not only meet compliance requirements but also enhance their operational efficiency and product safety.
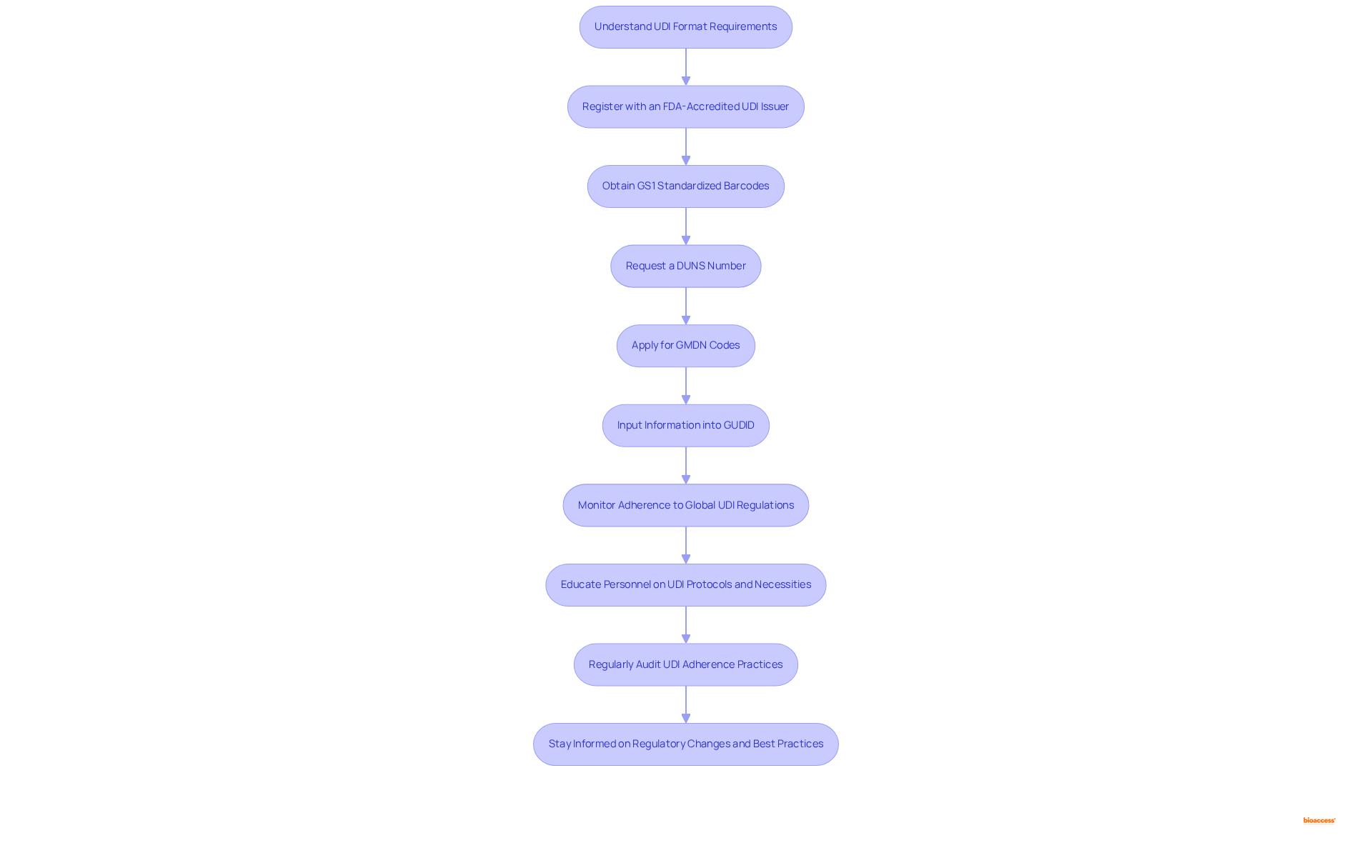
Achieving GUDID compliance is essential for medical device manufacturers aiming to navigate the complexities of regulatory requirements and ensure timely market access. This process involves a series of critical steps, from understanding the UDI format to accurately entering data into the GUDID database. Each stage plays a pivotal role in not only meeting compliance standards but also enhancing the overall safety and effectiveness of medical devices in the market.
Key arguments outlined in the article highlight the importance of:
The article emphasizes the necessity of meticulous data entry into the GUDID database, as well as the importance of continuous monitoring and training to maintain compliance with evolving regulations.
Ultimately, the benefits of adhering to GUDID compliance extend beyond regulatory requirements; they significantly enhance patient safety and trust in medical devices. By prioritizing UDI compliance, manufacturers not only streamline their operational processes but also contribute to a more reliable healthcare environment. Engaging with experts and utilizing resources like bioaccess® can further ease this journey, ensuring that manufacturers are well-equipped to meet compliance deadlines and improve patient outcomes. Taking proactive steps now will pave the way for successful market entry and foster a culture of safety and accountability in the medical device industry.
What is bioaccess® and how does it assist in GUDID compliance for medical devices?
bioaccess® accelerates adherence for medical products by utilizing its extensive knowledge of regulatory frameworks across Latin America, the Balkans, and Australia. It helps manufacturers navigate the complexities of registration, securing ethical approvals in 4-6 weeks and enhancing enrollment rates by 50%.
Why is regulatory adherence important for medical devices?
Regulatory adherence is critical for ensuring product safety and effectiveness in real-world settings. It influences the successful registration of medical devices, which is essential for timely market access.
What are the benefits of conducting clinical trials in Colombia?
Colombia offers cost efficiency for clinical trials, with savings of over 30% compared to North America and Western Europe. The regulatory review process typically spans 90-120 days, and the Colombian government provides R&D tax incentives, including a 100% tax deduction for investments in science and technology.
What is the Unique Device Identifier (UDI) format and its components?
The UDI format consists of two elements: the Device Identifier (DI), which identifies the labeler and specific version or model of the device, and the Production Identifier (PI), which provides additional details like the lot number and expiration date.
Why is mastering the UDI format important for manufacturers?
Mastering the UDI format is essential for accurately inputting equipment information into the GUDID, ensuring effective tracking and compliance, especially with the upcoming UDI/Device Registration period starting in Q4 2024 and culminating in a compliance deadline by Q2 2026.
What must producers do to meet UDI compliance standards?
Producers must enroll with an FDA-accredited UDI issuer to acquire a unique UDI for each medical item. This UDI must be displayed on the device label and packaging.
Which organizations are recognized as FDA-accredited UDI issuers?
The FDA recognizes several accredited issuing agencies, including GS1, HIBCC, and ICCBBA.
How does collaborating with an accredited UDI issuer benefit manufacturers?
Collaborating with an accredited issuer ensures compliance with FDA regulations and streamlines the process of entering the GUDID, leading to greater adherence rates and reduced risks of penalties and delays in market access.
According to the GMDN Agency, GMDN codes are used to assign each medical dev (https://mpo-mag.com/breaking-news/gmdn-codes-explainedaccording-to-the-gmdn-age)