


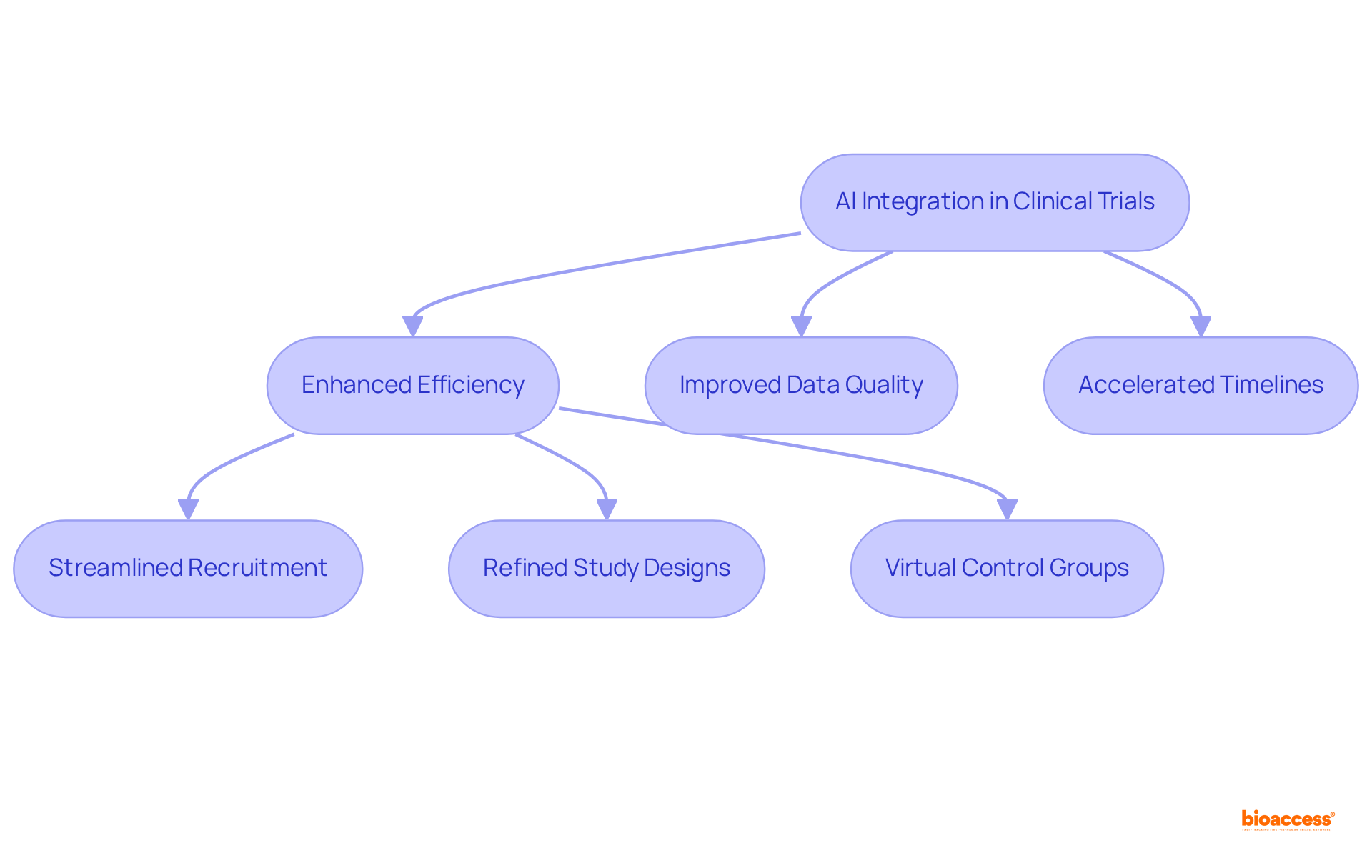
AI clinical trials data significantly transforms research efficiency by streamlining participant recruitment, enhancing data quality, and accelerating study timelines. This article highlights these transformative effects through various AI applications, including predictive analytics and virtual control groups. Collectively, these innovations reduce enrollment durations and improve the inclusivity of clinical research. Ultimately, this leads to the development of more effective therapies and better healthcare outcomes.
AI is reshaping the landscape of clinical research, ushering in a new era of efficiency and precision. By harnessing the power of AI clinical trials data, researchers can streamline processes, enhance participant diversity, and improve overall study outcomes. However, as these transformative technologies gain traction, pressing questions arise:
Exploring these dynamics reveals not only the potential benefits of AI in clinical trials but also the challenges that must be navigated to fully realize its promise.
bioaccess® harnesses AI clinical trials data and AI-driven insights to optimize clinical study processes, significantly accelerating timelines for ethical approvals and participant enrollment. By leveraging the capabilities of C2-Ai's Tracking List (PTL) tool, bioaccess® identifies the most effective trial designs and target demographics. This results in trials that are not only swifter but also more efficient. Such an innovative approach has been proven to reduce the average duration of stay for individuals, with reports indicating:
Furthermore, assessments from NHS England reveal that this integration of AI has freed up 125 bed-days per 1,000 individuals, thereby enhancing resource allocation. The incorporation of AI clinical trials data also elevates the quality of data collected, leading to more reliable outcomes and ultimately propelling the pace of medical innovation. As Professor Rowan Pritchard-Jones remarked, 'I saw waiting lists balloon through the pandemic... and asked if we could get a forward-looking report predicting patient need.' This underscores the pivotal role of AI in enhancing research efficiency.
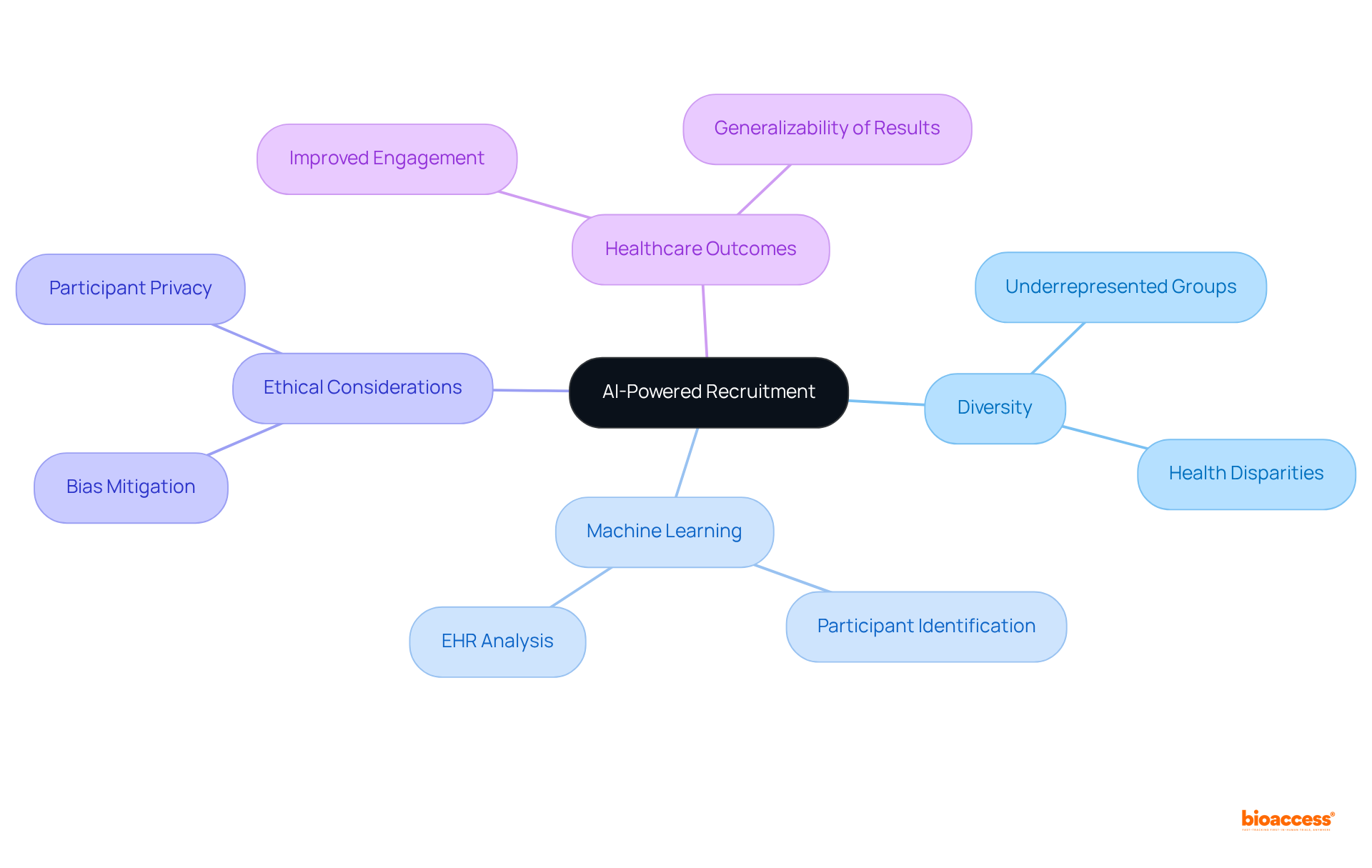
AI-driven recruitment tools harness extensive AI clinical trials data to effectively identify and engage diverse participant pools. By employing advanced machine learning algorithms, researchers can connect potential participants with studies that align with their profiles, significantly enhancing enrollment rates among underrepresented groups. As Bin Zhang noted, "AI can be utilized to guide research eligibility standards, improve participant diversity, and decrease sample size needs." This strategy not only fosters inclusivity within medical studies but also enhances the generalizability of results, ultimately contributing to improved healthcare outcomes across diverse demographics.
The importance of participant variety in medical studies cannot be overstated; it ensures that research outcomes are relevant to a broader population, addressing health disparities and promoting equitable healthcare solutions. Furthermore, the increasing interest in AI applications is evidenced by successful training programs that have engaged nearly 100 participants, leading to their recommissioning for additional cohorts in 2023. Additionally, AI's capability to analyze electronic health records (EHRs) enables researchers to identify eligible participants more effectively, as highlighted in the case study 'AI Clinical Trials Data for Participant Identification.'
However, it is crucial to address the ethical implications of AI in clinical studies, ensuring that tools are programmed without biases and that participant privacy is safeguarded.
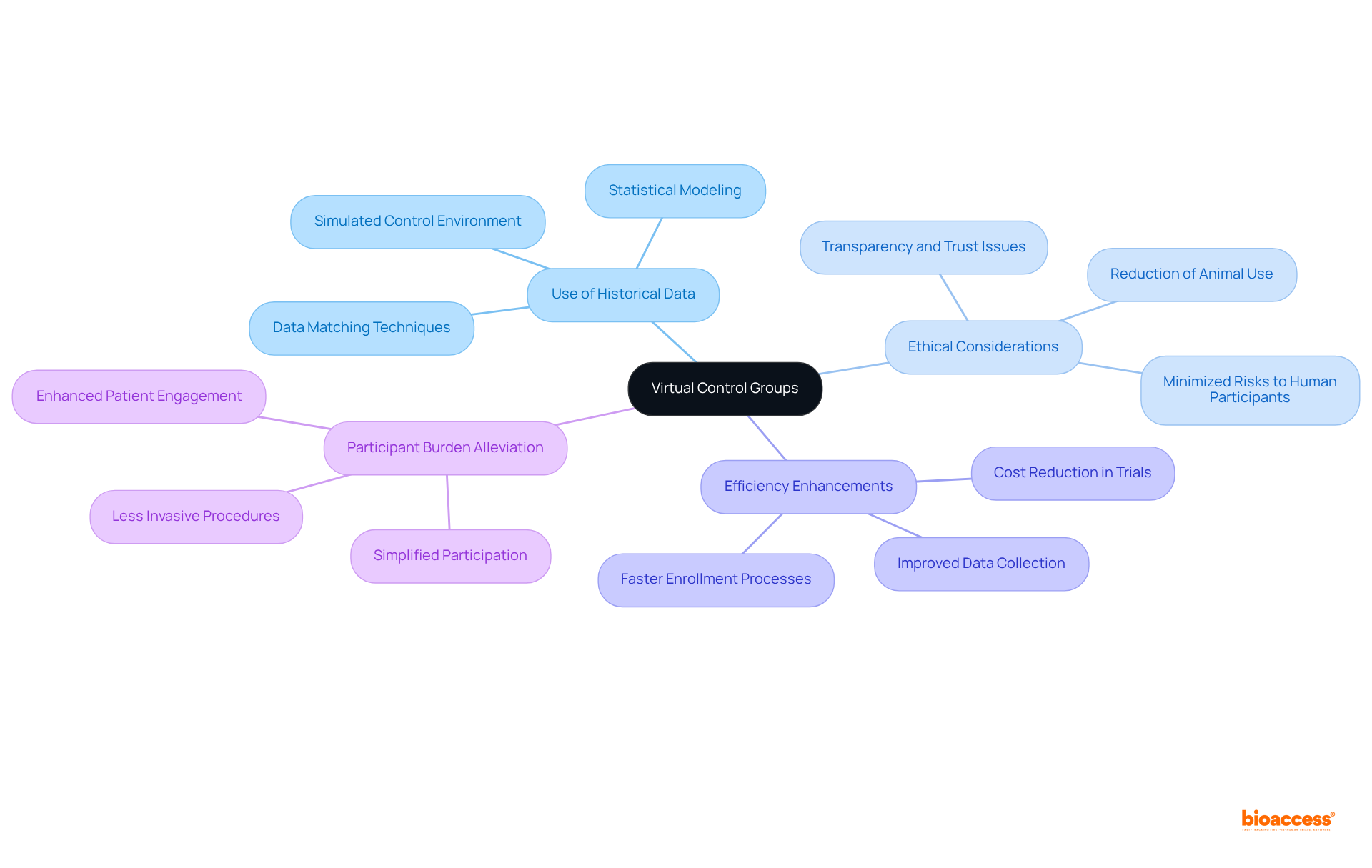
Virtual control groups leverage historical data to create a simulated control environment, allowing researchers to conduct experiments free from the ethical dilemmas tied to traditional control groups. By employing AI algorithms to analyze AI clinical trials data, these virtual groups facilitate more accurate comparisons and expedited results. This groundbreaking approach not only enhances the efficiency of research studies but also alleviates participant burden and mitigates ethical concerns associated with control groups. In the evolving Medtech landscape, the adoption of virtual control groups, supported by AI clinical trials data, represents a significant advancement in addressing key challenges within clinical research.
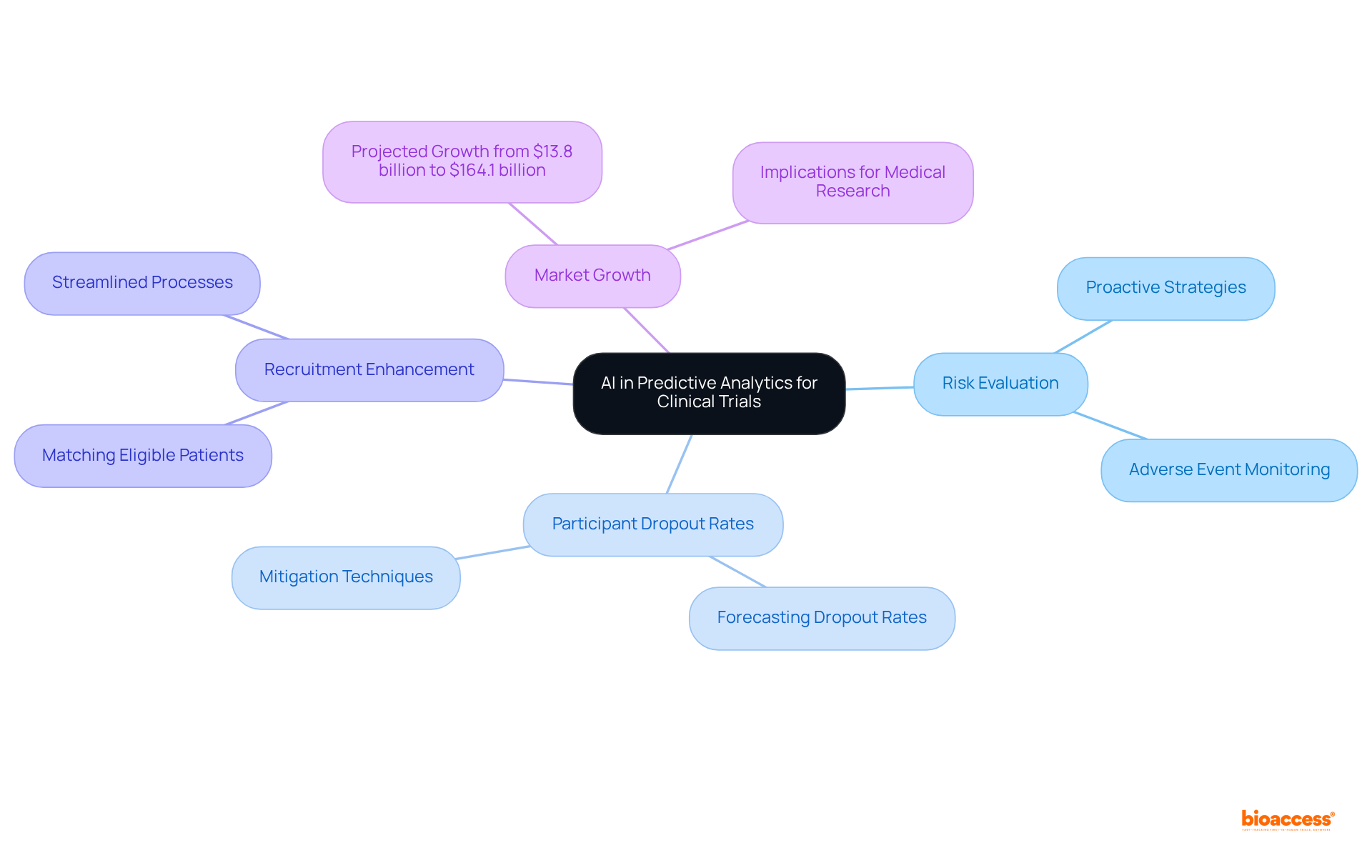
Predictive analytics powered by AI clinical trials data significantly transforms risk evaluation in research studies by utilizing historical data to detect patterns that indicate potential challenges. This advanced technology empowers researchers to forecast issues such as participant dropout rates and adverse events, enabling the implementation of proactive strategies to mitigate these risks.
For instance, AI tools can analyze extensive datasets to enhance participant recruitment by aligning qualified patients with studies, which substantially reduces dropout rates. Furthermore, the integration of AI not only bolsters the safety of research studies but also enhances overall study efficiency and success rates.
The market for AI in healthcare is projected to surge from $13.8 billion in 2022 to an astonishing $164.1 billion by 2029, underscoring the profound implications for medical research. By adopting AI-driven predictive analytics, organizations can streamline processes and enhance decision-making, ultimately accelerating the path to successful outcomes based on AI clinical trials data.
AI technologies are revolutionizing the analysis of AI clinical trials data in clinical research by automating critical processes such as collection, cleaning, and interpretation. Advanced algorithms swiftly analyze complex datasets, uncovering insights that would typically demand extensive time and effort from human analysts. This optimization accelerates research timelines and significantly enhances accuracy. Organizations employing AI have reported notable improvements in information integrity and reliability, which are essential for developing effective treatments and therapies.
By 2025, the integration of AI in medical studies is expected to further enhance information precision, ensuring that results are both robust and applicable. Automated data collection methods, including real-time monitoring and sensor integration, illustrate how AI can streamline research efforts, facilitating more efficient and precise data handling. These advancements in AI clinical trials data are paving the way for a new era in clinical studies, where speed and precision are paramount.
AI-driven platforms significantly enhance patient engagement by delivering personalized communication and educational resources specifically tailored to individual participant needs. These innovative tools, including chatbots and automated messaging systems, can effectively respond to inquiries, provide timely reminders, and supply crucial information. This ensures that participants remain informed and engaged throughout the research process.
Such a proactive strategy not only elevates participant satisfaction but also substantially boosts retention rates, thereby contributing to the overall success of research studies. In the evolving landscape of clinical research, leveraging these technologies is essential for fostering collaboration and achieving optimal outcomes.
The incorporation of AI clinical trials data in clinical studies presents considerable ethical dilemmas, particularly regarding information privacy, informed consent, and algorithmic bias. As AI technologies evolve, researchers must navigate these complexities by establishing robust ethical frameworks and ensuring transparency in their applications, especially regarding AI clinical trials data. Notably, in 2025, data privacy challenges are anticipated to intensify, necessitating stringent measures to protect sensitive patient information.
Clinical study sponsors should prioritize informed consent procedures that clearly convey how AI will be utilized, thereby fostering participant trust and engagement. Furthermore, addressing algorithmic bias is crucial to ensure equitable outcomes across diverse populations. By adopting these ethical practices, sponsors can enhance the credibility of their research, ultimately leading to more successful and reliable study outcomes. This proactive approach not only safeguards participant rights but also aligns with current trends that emphasize the importance of ethical AI deployment in healthcare.
The advancement of AI technology is set to revolutionize clinical research, with AI clinical trials data and generative AI poised to play a pivotal role in trial design by 2025. This innovative approach facilitates the simulation of various outcomes, optimizing study protocols and enhancing overall efficiency.
Moreover, the increasing use of wearable devices is noteworthy, as research indicates that their integration can significantly improve monitoring and data collection precision. For instance, wearables allow for continuous tracking of health metrics, providing real-time insights into participant well-being.
As these technologies evolve, the incorporation of real-world information will further refine testing protocols, ensuring they align more closely with individual requirements. Collectively, these advancements are anticipated to enhance the quality of AI clinical trials data collected, ultimately leading to improved patient outcomes and more effective studies.
Integrating AI with conventional research methodologies fosters a collaborative environment that significantly enhances the efficiency of studies. By leveraging the strengths of AI—such as data analysis and predictive techniques—alongside the expertise of research professionals, teams can develop more robust study designs and improve decision-making processes. This powerful synergy accelerates research timelines while elevating the quality of findings, ultimately serving to advance healthcare outcomes.
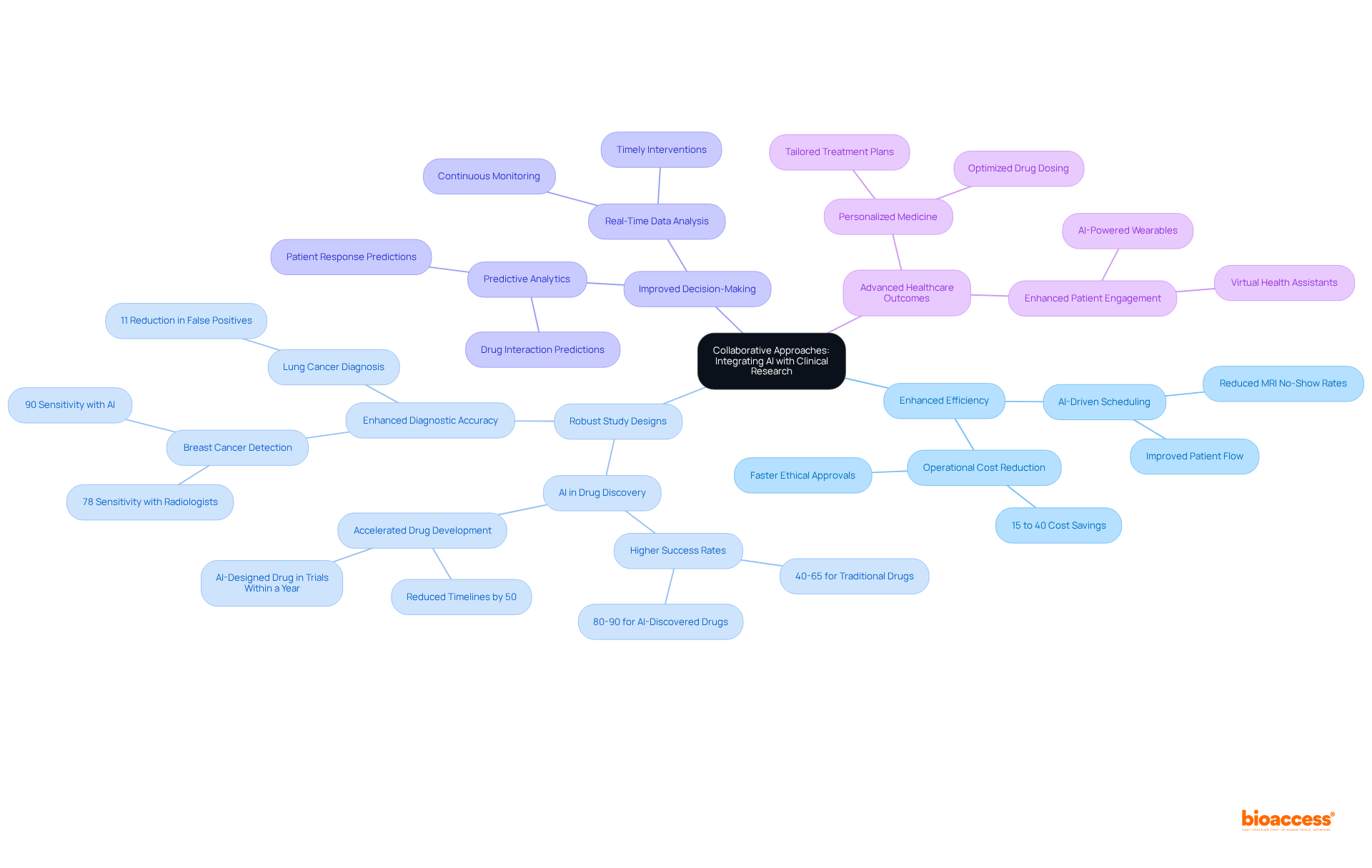
AI clinical trials data is fundamentally transforming research studies by significantly enhancing efficiency, improving data quality, and accelerating timelines. Cutting-edge AI algorithms streamline participant recruitment using AI clinical trials data, which greatly reduces recruitment durations, while predictive analytics refine study designs and site selection. The introduction of virtual control groups—a crucial component of decentralized research studies (DCTs)—further enhances the inclusivity and diversity of medical investigations, addressing historical challenges in participant representation.
As these technologies evolve, they promise to deliver more effective therapies and improve outcomes for individuals, marking a significant advancement in research methodologies. For instance, organizations leveraging AI have reported up to a 40% reduction in trial enrollment durations, illustrating the tangible benefits of these innovations. The integration of AI clinical trials data not only expedites drug discovery but also ensures that the landscape of clinical research is increasingly responsive to the needs of patients and healthcare providers.
As Bhavik Shah from McKinsey asserts, "Pharma companies would be unwise not to recognize the transformative potential of AI.
AI clinical trials data is revolutionizing the landscape of medical research by enhancing efficiency, improving data quality, and accelerating the timelines necessary for successful outcomes. The integration of advanced AI technologies empowers researchers to streamline processes—from participant recruitment to risk assessment—ultimately fostering a more responsive and effective clinical trial environment.
Key insights from the article highlight the multifaceted benefits of AI in clinical trials:
Together, these innovations not only reduce enrollment durations but also enhance the overall quality of research findings.
As the role of AI continues to evolve, it is imperative for stakeholders in the healthcare sector to embrace these transformative technologies. By prioritizing ethical considerations and fostering collaboration between AI and traditional research methodologies, the future of clinical trials promises to deliver more inclusive, efficient, and effective medical solutions. The advancements in AI clinical trials data will undoubtedly shape a new era in healthcare, making it crucial for organizations to adapt and innovate in response to these emerging trends.
What is bioaccess® and how does it improve clinical trials?
bioaccess® utilizes AI clinical trials data and insights to optimize clinical study processes, significantly speeding up timelines for ethical approvals and participant enrollment. It identifies effective trial designs and target demographics, leading to more efficient trials.
What benefits have been reported from using the PTL tool in clinical trials?
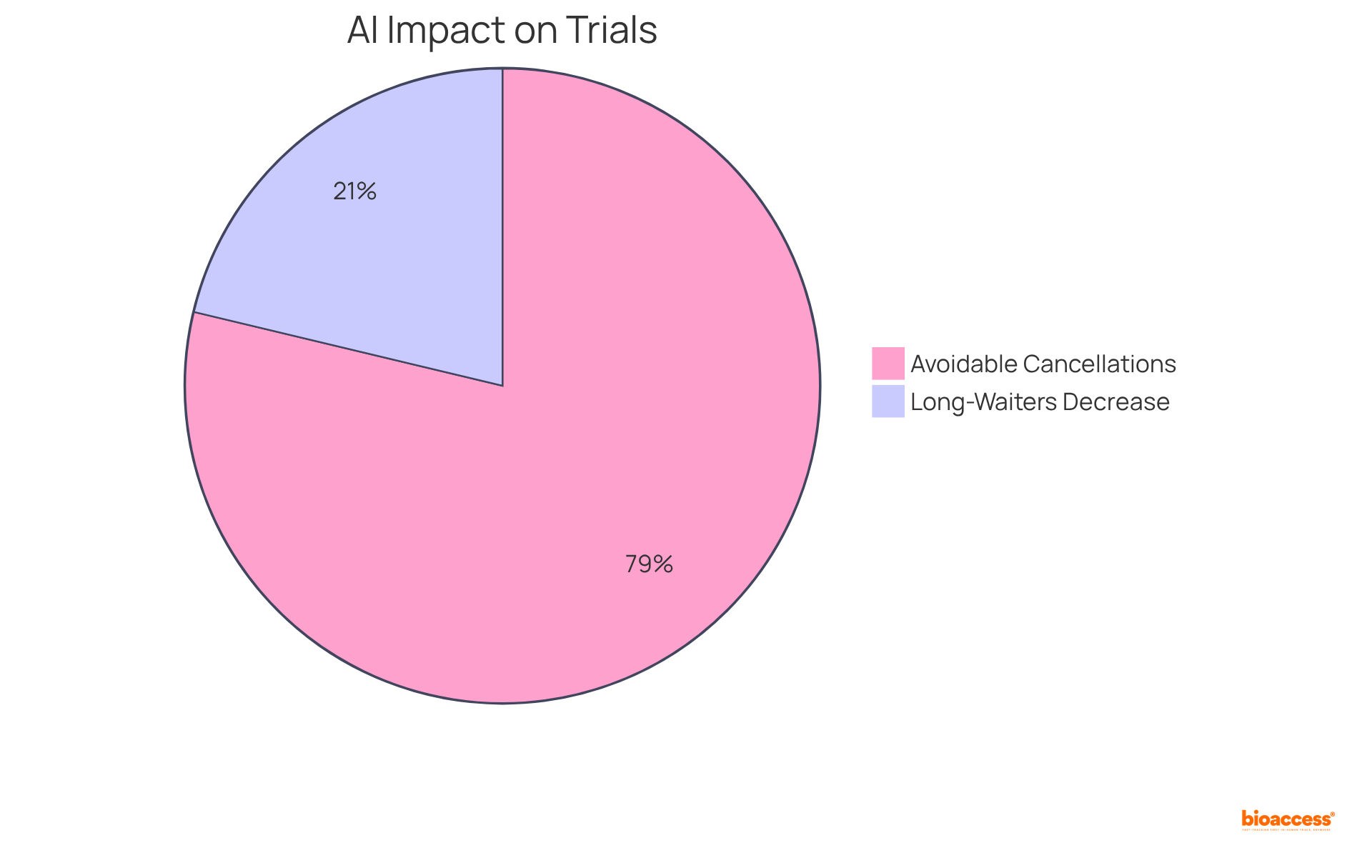
The PTL tool has resulted in a 27% decrease in long-waiters and a 100% reduction in avoidable cancellations, contributing to shorter trial durations and enhanced resource allocation.
How does AI integration impact resource allocation in clinical trials?
Assessments from NHS England indicate that the integration of AI has freed up 125 bed-days per 1,000 individuals, improving the allocation of healthcare resources.
What role does AI play in participant recruitment for clinical trials?
AI-driven recruitment tools utilize extensive clinical trials data to identify and engage diverse participant pools, improving enrollment rates among underrepresented groups and enhancing the generalizability of research outcomes.
Why is participant diversity important in clinical trials?
Participant variety ensures that research outcomes are relevant to a broader population, addressing health disparities and promoting equitable healthcare solutions.
What ethical considerations are associated with AI in clinical studies?
It is essential to ensure that AI tools are programmed without biases and that participant privacy is safeguarded during the recruitment and data analysis processes.
What are virtual control groups and how do they benefit clinical trials?
Virtual control groups use historical data to create a simulated control environment, allowing researchers to conduct studies without the ethical dilemmas of traditional control groups. This approach enhances efficiency and reduces participant burden.
How do AI algorithms contribute to the use of virtual control groups?
AI algorithms analyze clinical trials data to facilitate accurate comparisons and expedited results, improving the overall design and effectiveness of clinical research studies.