


The essential features of electronic data capture (EDC) software are critical to enhancing clinical research efficiency. These features include:
Collectively, they significantly enhance the efficiency, accuracy, and integrity of clinical trials. By streamlining data collection, ensuring regulatory compliance, and improving user experience, these capabilities are underscored by various case studies and industry insights throughout the article.
In the fast-paced realm of clinical research, the efficiency and accuracy of data management are pivotal to the success of any study. Electronic Data Capture (EDC) software has emerged as a transformative solution, providing a comprehensive suite of essential features that streamline the collection, analysis, and reporting of clinical trial data. This article explores the eight critical functionalities of EDC systems, illustrating how they not only enhance operational efficiency but also protect the integrity of research outcomes. As the adoption of these advanced technologies accelerates, it is crucial to consider the challenges researchers face in fully harnessing their potential.
At bioaccess®, the implementation of accelerated electronic data capture software significantly enhances the efficiency of clinical trials. This innovative approach ensures that information is collected, managed, and analyzed swiftly, facilitating quicker decision-making and improving patient outcomes.
With advanced EDC solutions, bioaccess® not only reduces the time required for data input but also minimizes errors, thereby guaranteeing high-quality information essential for regulatory submissions and analysis.
In the rapidly evolving Medtech landscape, such advancements are crucial in addressing key challenges faced by clinical researchers, underscoring the importance of collaboration in achieving superior results.
An intuitive user interface in electronic data capture software is essential for optimizing user experience and operational efficiency. By facilitating smooth navigation, researchers and entry staff can significantly reduce the learning curve, leading to increased productivity. Essential features such as:
contribute to a seamless experience. This design approach allows users to concentrate on ensuring quality rather than grappling with software complexities. Case studies illustrate that organizations implementing user-centric designs in their electronic data capture software experience smoother workflows and improved data accuracy, ultimately elevating the overall standard of research. Furthermore, professional insights indicate that a well-crafted interface fosters greater user satisfaction, which is vital for the successful adoption of research software. As the transition to electronic data capture software systems continues to reshape medical studies, with enrollment occurring 50% faster than in traditional markets, it will be essential to prioritize user experience through thoughtful interface design to maximize the benefits of these advanced technologies. As noted by Datavant,
'CDM plays a crucial role in the advancement of new medications and medical instruments, ensuring that the information utilized in research is both precise and appropriate for evaluation.'
Strong security protocols are essential in electronic data capture software to protect clinical trial details. Key strategies include the encryption of information both at rest and in transit, which is crucial for protecting sensitive patient data. Regulated entities must implement reasonable safeguards to protect electronic protected health information (ePHI), incorporating technical measures to guard against unauthorized access, as mandated by the Security Rule of HIPAA and the HITECH Act.
Secure user authentication protocols are also crucial, ensuring that only authorized personnel can access sensitive information. Regular security audits further enhance protection by identifying vulnerabilities and ensuring compliance with regulatory standards. These audits help verify the identity of individuals seeking access to ePHI and establish audit controls to monitor system activity.
Recent violations in research security have underscored the necessity for strict measures. For instance, the HITECH Act has strengthened privacy and security protections for health information, making business associates directly liable for violations of the Security Rule. This highlights the importance of comprehensive security policies and procedures, including the necessity for regulated entities to enter into written contracts or business associate agreements to safeguard ePHI.
The effect of strong information protection on research integrity cannot be overstated. Case studies demonstrate that organizations with robust security measures not only safeguard patient information but also enhance the overall trustworthiness of their research. By emphasizing information security, bioaccess® guarantees the confidentiality, integrity, and availability of research information, ultimately promoting trust and dependability in the research process. Organizations are encouraged to regularly review and update their security policies in response to changes affecting the security of ePHI.
Real-time information access significantly transforms clinical studies by providing researchers with instantaneous insights into study progress and participant reactions. The electronic data capture software continuously updates data, enabling the rapid identification of trends or issues that may require intervention. This capability not only enhances the responsiveness of research teams but also substantially improves the overall quality of results.
Shorter study durations, attributed to real-time monitoring, can lead to increased participation rates and a more diverse participant pool, resulting in robust outcomes. As one clinical investigator noted, "The ability to obtain real-time information enables Clinical Research Organizations (CROs) to gain prompt insights for rapid protocol modifications," ultimately fostering a more efficient and effective process.
Furthermore, the integration of sophisticated analytics within electronic data capture software enhances decision-making, ensuring that researchers can respond to insights as they arise, thereby improving performance and results. Additionally, real-time observation reduces reliance on self-reported information, thereby enhancing accuracy and supporting the integrity of the study.
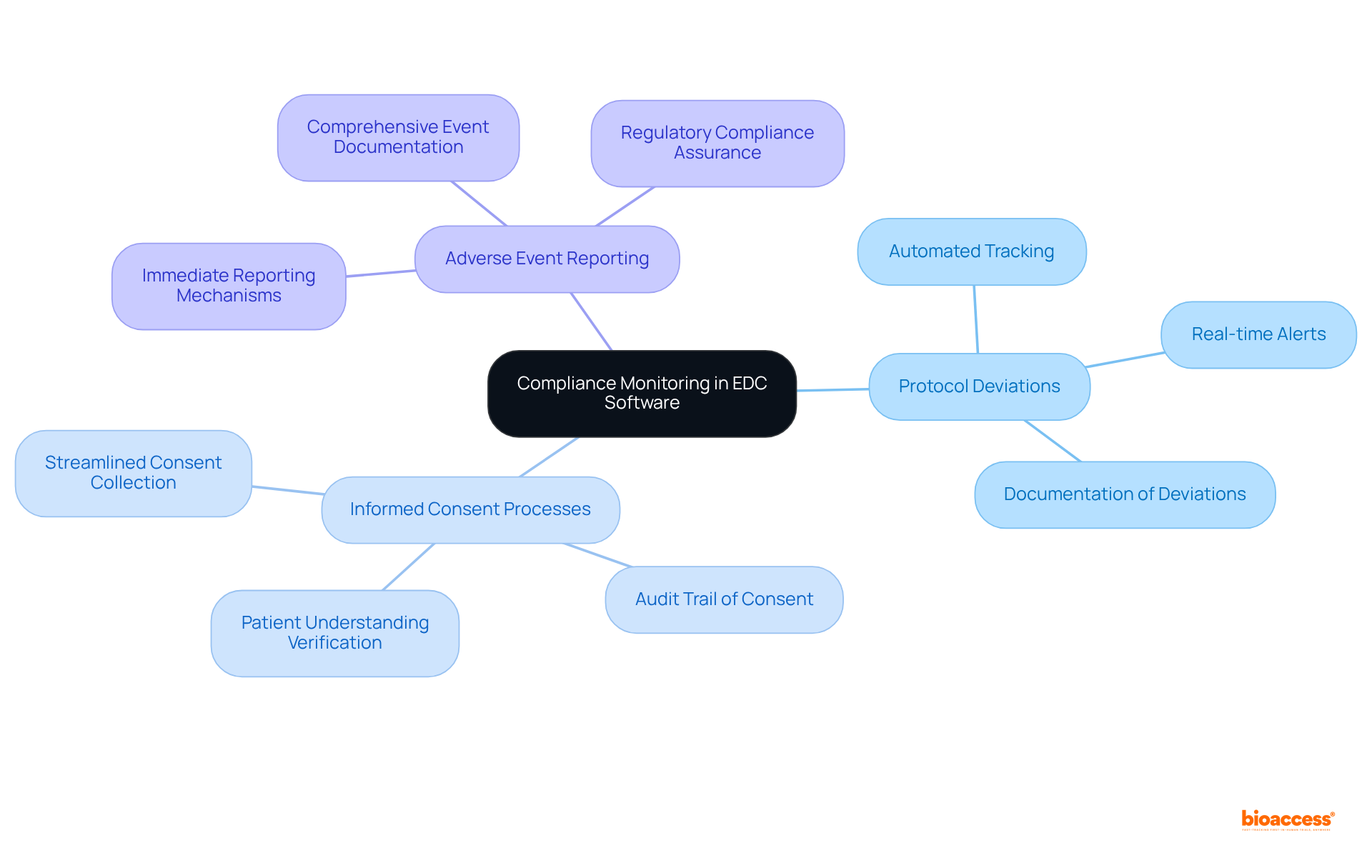
Compliance monitoring functions in EDC software are essential for ensuring that research studies meet regulatory standards. These features facilitate automated tracking of:
By maintaining a comprehensive audit trail, bioaccess® effectively demonstrates compliance to regulatory bodies. This capability not only facilitates smoother approvals but also enhances the credibility of the research conducted.
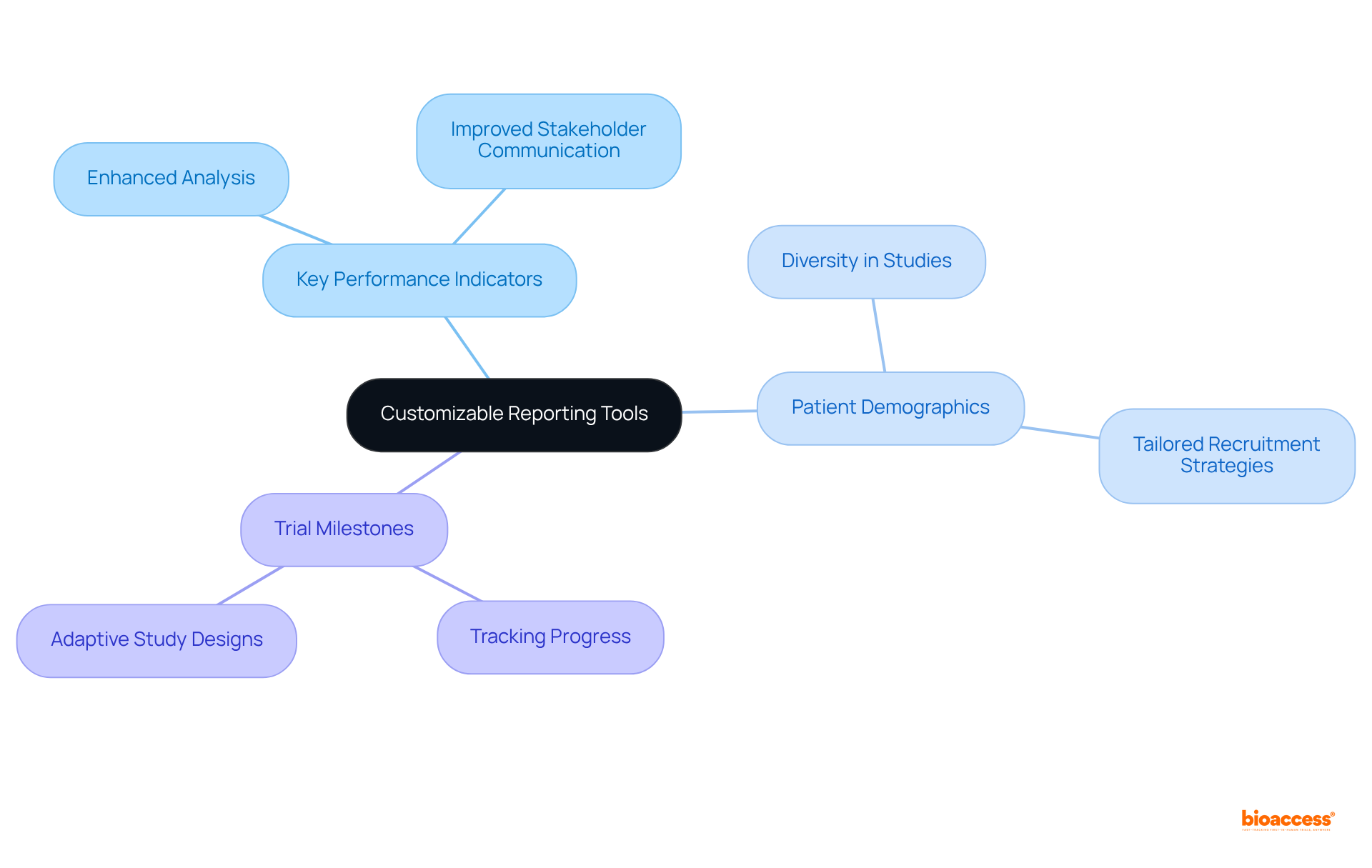
Customizable reporting tools in EDC software are pivotal for researchers aiming to generate insights tailored to their specific needs. These tools enable users to create reports focused on key performance indicators, patient demographics, or trial milestones, thereby enhancing both analysis and presentation. This flexibility not only empowers research teams to communicate findings effectively to stakeholders but also fosters transparency and collaboration, which are critical in the evolving landscape of clinical research that can be enhanced through electronic data capture software.
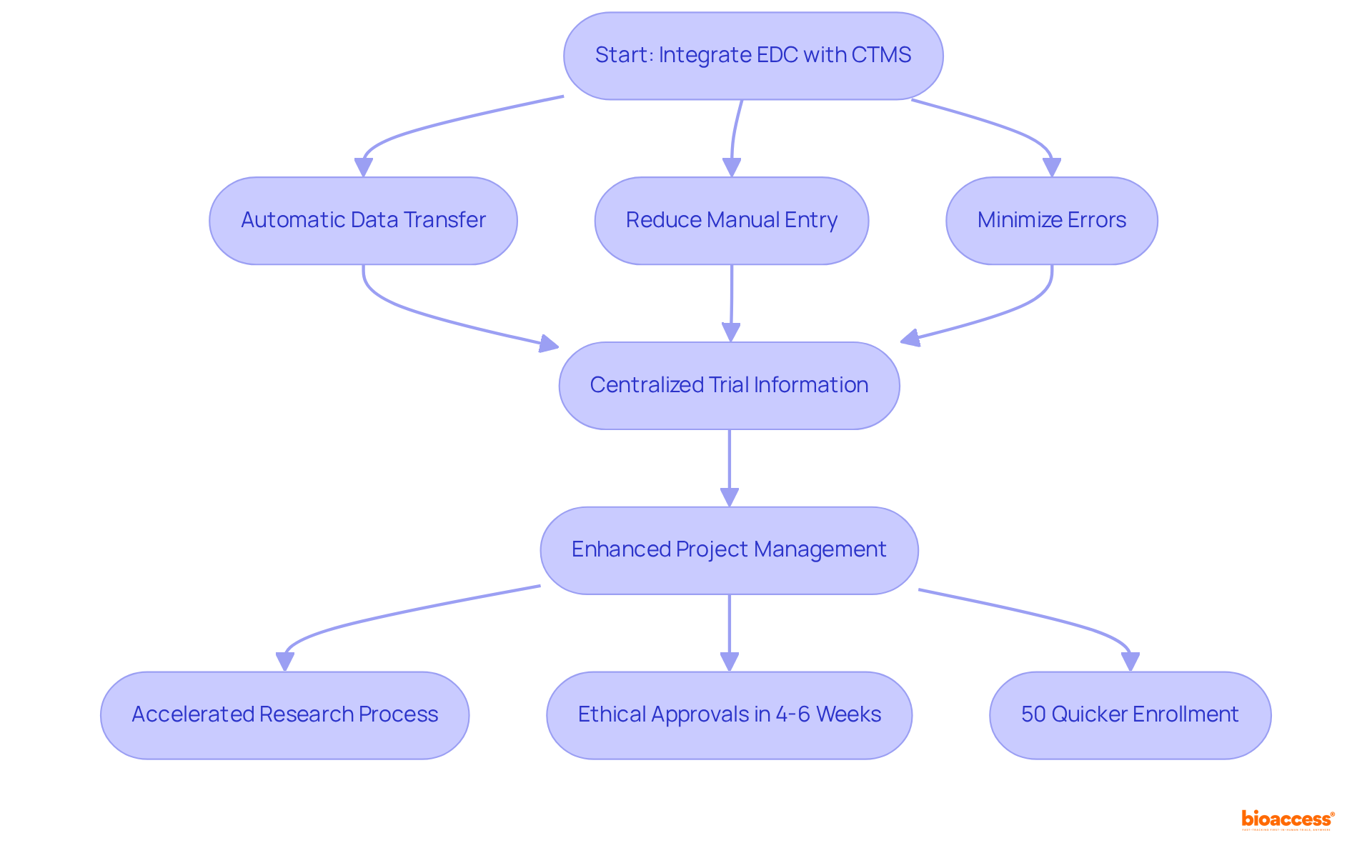
The seamless integration of electronic data capture software with Clinical Trial Management Systems (CTMS) is essential for enhancing operational efficiency in research. This integration of electronic data capture software allows for the automatic transfer of data between systems, significantly reducing the need for manual data entry and minimizing the risk of errors. By connecting the electronic data capture software with CTMS, bioaccess® centralizes all trial-related information, which enhances project management and oversight. This streamlined method not only accelerates the research process but also underscores bioaccess®'s commitment to providing ethical approvals in just 4-6 weeks and achieving enrollment rates that are 50% quicker than conventional markets.
Moreover, bioaccess® specializes in early-phase research services for the Medtech, Biopharma, and Radiopharma industries, linking innovative companies with the opportunities to conduct research in Latin America, the Balkans, and Australia. Additionally, bioaccess® offers market access services that empower clients to leverage Latin America’s multi-billion dollar healthcare market, further bolstering their efforts to advance products swiftly and effectively.
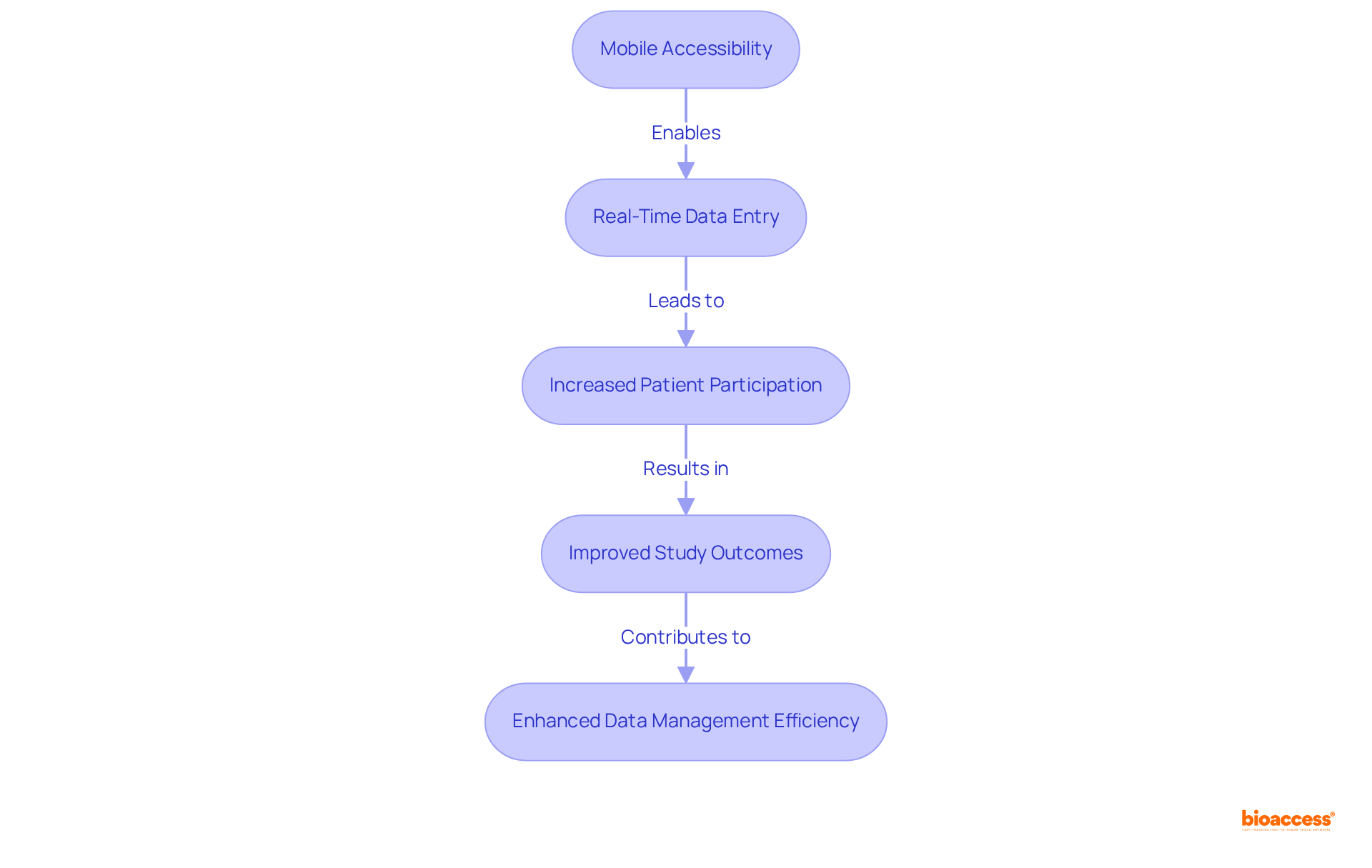
The adaptability of information gathering in clinical trials is significantly enhanced by mobile accessibility in electronic data capture software. This capability proves especially advantageous for research conducted in remote areas or with mobile patient populations, as it facilitates real-time data entry and monitoring. By enabling information collection through mobile devices, bioaccess® ensures high participant engagement and accurate, timely data capture.
Researchers have noted that mobile data gathering not only streamlines the process but also fosters increased patient participation, ultimately leading to improved study outcomes. A systematic review reveals that only 20% of medical studies are utilizing electronic data capture software, highlighting the urgent need for advancement in this domain.
Furthermore, the FDA's 2023 recommendations on decentralized research highlight the critical role of mobile data collection in adapting to the evolving regulatory landscape. The integration of mobile technology in clinical research is becoming increasingly essential, as it addresses the complexities of modern trials and enhances the overall efficiency of data management.
The automated information validation features in electronic data capture software significantly enhance integrity by ensuring that all entered information adheres to predefined criteria. This proactive approach enables the real-time detection of errors or inconsistencies, which is essential for upholding high quality standards. By managing large datasets simultaneously, batch validation improves efficiency, allowing for prompt corrections that are crucial for trustworthy research outcomes.
In a large-scale medical study, for instance, the use of automated validation tools led to a significant decrease in entry mistakes and enhanced overall quality, thereby promoting smoother regulatory approval processes. Furthermore, information accuracy in medical studies is vital as it guarantees dependability and adherence to regulations.
Frequent evaluations and reconciliation procedures, as illustrated in a centralized information monitoring case study from a multi-site research study, strengthen consistency and precision, underscoring the necessity for robust validation mechanisms. The three primary elements of information validation—Accuracy, Completeness, and Consistency—are fundamental to this process.
By leveraging these automated features, bioaccess® not only maintains the integrity of research information through electronic data capture software but also enhances the overall efficiency of the investigative process.
The effective utilization of electronic data capture software is fundamentally reliant on comprehensive training and robust support systems. bioaccess® offers extensive training programs that encompass everything from basic navigation to advanced information management techniques, ensuring that users are thoroughly prepared to leverage the software's full potential.
Statistics indicate that organizations with well-structured training programs experience a notable increase in EDC utilization; nearly 70% of biotech firms report difficulties in integrating EDC technology with existing systems due to inadequate training. This underscores the critical role that comprehensive training plays in overcoming these challenges.
Moreover, ongoing support is essential, empowering users to troubleshoot issues and optimize their system use. This dual approach not only enhances data quality but also significantly improves trial efficiency.
Insights from clinical research leaders emphasize that effective management of informed consent and data integrity is crucial for regulatory compliance and safeguarding participant rights. Chris Rush highlights that managing informed consent throughout these processes is vital for upholding participant rights.
Additionally, investing in comprehensive training and support can yield cost savings ranging from 49% to 62% compared to traditional documentation methods, thereby maximizing the capabilities of EDC systems and ultimately driving superior research outcomes.
The implementation of electronic data capture (EDC) software is revolutionizing the landscape of clinical trials, significantly enhancing efficiency and data integrity. By streamlining processes such as data entry, security, and compliance tracking, EDC systems like those offered by bioaccess® empower researchers to focus on what truly matters—delivering high-quality outcomes and advancing medical research.
Key features of EDC software include:
These functionalities play a vital role in optimizing the research process. They not only facilitate smoother workflows and enhance data accuracy but also ensure that compliance with regulatory standards is maintained throughout the trial. Moreover, the seamless integration of EDC with clinical trial management systems (CTMS) and the provision of mobile accessibility further enhance operational efficiency, allowing for timely data collection and analysis.
The significance of adopting advanced EDC solutions cannot be overstated. As the clinical research environment continues to evolve, leveraging these technologies will be crucial for organizations aiming to improve patient outcomes and accelerate the development of new treatments. Embracing comprehensive training and support for EDC software will also ensure that research teams are well-equipped to maximize the potential of these tools, ultimately driving superior results in clinical trials.
What is bioaccess® and how does it enhance clinical trials?
bioaccess® implements accelerated electronic data capture software that significantly improves the efficiency of clinical trials by ensuring swift collection, management, and analysis of data, leading to quicker decision-making and better patient outcomes.
How does bioaccess® reduce errors in data capture?
The advanced EDC solutions provided by bioaccess® minimize the time required for data input and reduce errors, ensuring high-quality information that is essential for regulatory submissions and analysis.
What role does the intuitive user interface play in EDC software?
An intuitive user interface in electronic data capture software optimizes user experience and operational efficiency by facilitating smooth navigation, which reduces the learning curve and increases productivity.
What are some key features of an intuitive user interface in EDC software?
Key features include drag-and-drop functionality, clearly defined navigation menus, and customizable dashboards, all contributing to a seamless user experience.
How does strong data security benefit clinical trials?
Strong security protocols protect sensitive clinical trial information through strategies like encryption of data, secure user authentication, and regular security audits, ensuring compliance with regulations and safeguarding patient data.
What regulations govern the security of electronic protected health information (ePHI)?
The Security Rule of HIPAA and the HITECH Act mandate regulated entities to implement safeguards to protect ePHI, including technical measures against unauthorized access.
Why are regular security audits important in clinical trials?
Regular security audits identify vulnerabilities, ensure compliance with regulatory standards, verify identities of individuals accessing ePHI, and establish audit controls to monitor system activity.
How does bioaccess® ensure the confidentiality and integrity of research information?
By emphasizing robust security measures, bioaccess® guarantees the confidentiality, integrity, and availability of research information, which promotes trust and dependability in the research process.
What should organizations do to maintain strong information security?
Organizations are encouraged to regularly review and update their security policies in response to changes affecting the security of ePHI to maintain strong information protection.