


The article titled "8 Key Insights on 21 CFR Part 820 FDA Compliance for Medtech Leaders" primarily aims to equip Medtech leaders with essential guidance in navigating FDA compliance, specifically concerning the Quality System Regulation (QSR) under 21 CFR Part 820. It underscores the significance of:
These are pivotal strategies that not only enhance operational efficiency but also ensure compliance with regulatory standards. These measures ultimately facilitate smoother FDA inspections and expedite market access for medical devices.
Navigating the intricate landscape of FDA compliance is a critical undertaking for Medtech leaders, particularly in light of the evolving regulations outlined in 21 CFR Part 820. This framework serves not only as a foundation for ensuring product safety and efficacy but also presents a unique opportunity for manufacturers to enhance their operational efficiency and market readiness. However, as the FDA intensifies its scrutiny and introduces new inspection processes, the pressing question arises: how can Medtech companies effectively prepare to meet these stringent requirements while maintaining a competitive edge in a rapidly changing industry?
bioaccess® leverages the regulatory agility of Latin America, the diverse patient demographics of the Balkans, and the efficient routes of Australia to accelerate FDA adherence for Medtech innovators. With ethical approvals achieved in a mere 4-6 weeks and enrollment processes that are 50% faster than traditional markets, bioaccess® significantly enhances companies' capabilities to swiftly bring their innovations to market. This expedited approach not only meets FDA standards effectively but also aligns with the growing demand for quicker evaluations in the Medtech sector.
Industry leaders assert that regulatory speed is vital for maintaining a competitive edge, enabling companies to swiftly respond to market needs and seize emerging opportunities. By implementing effective adherence strategies, such as meticulous documentation and proactive engagement with regulatory bodies, Medtech firms can navigate the complexities of the FDA submission process while ensuring compliance with 21 CFR Part 820 FDA more efficiently.
bioaccess® stands out as a dedicated partner, committed to expediting the journey from idea to market, ensuring that innovations reach patients more swiftly while upholding the highest standards of regulatory adherence.

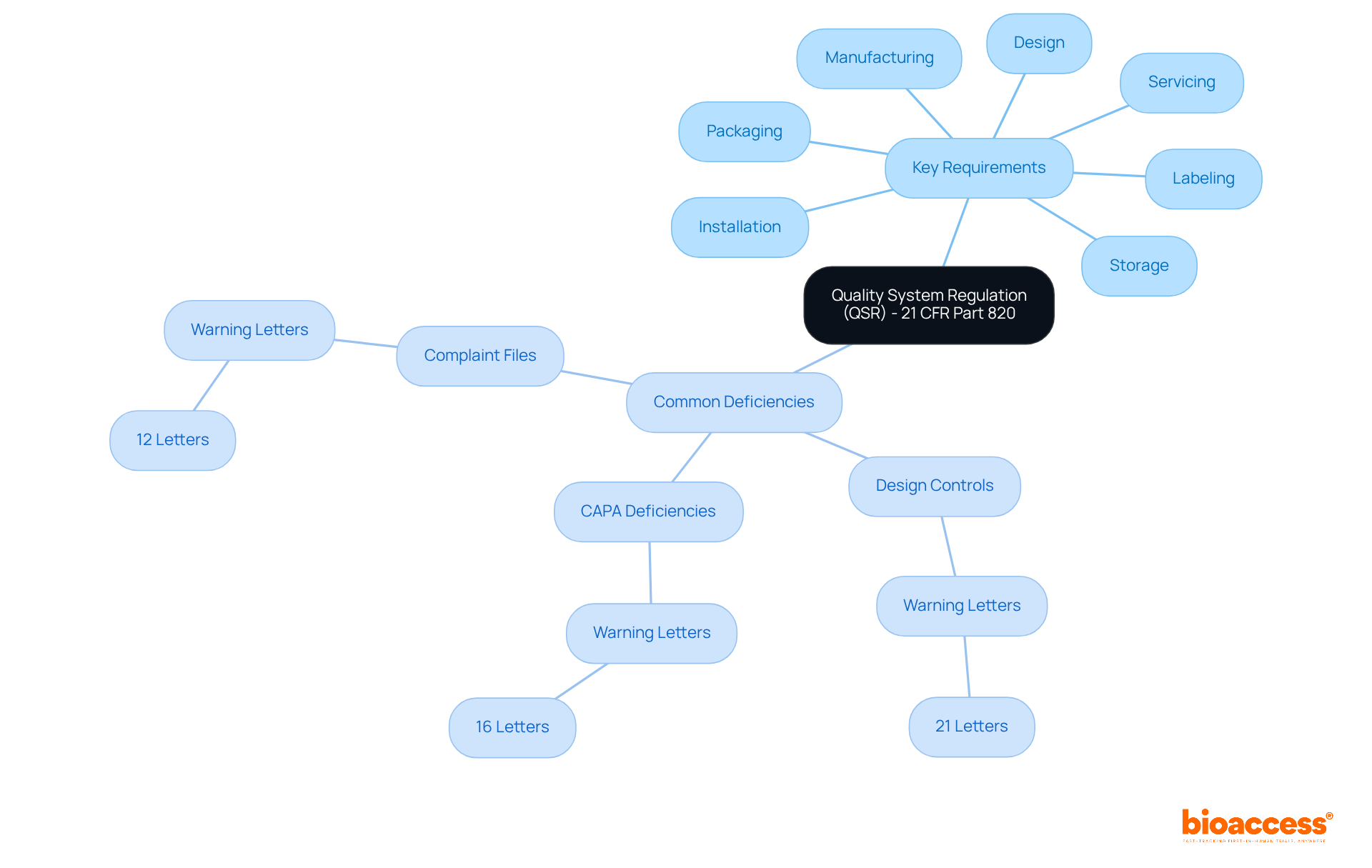
The Quality System Regulation (QSR) outlined in 21 CFR Part 820 FDA serves as the cornerstone for a robust Quality Management System (QMS) that medical device manufacturers must implement. This regulation encompasses a wide array of requirements, including:
Adhering to the QSR is essential for ensuring that products are both safe and effective, a prerequisite for obtaining FDA approval.
In the fiscal year 2024, the FDA issued a total of 47 Warning Letters to medical device manufacturers, a significant increase from 24 in the previous year. This emphasizes the ongoing challenges manufacturers encounter in upholding regulations. Common deficiencies include:
Experts stress that the QSR not only supports regulatory compliance but also improves product standards and safety. By creating thorough documentation and strict control procedures, manufacturers can effectively reduce risks linked to human error. For example, AI can streamline processes in management systems to improve product standards and minimize risks associated with human mistakes. The execution of automated document control systems has shown advantageous in attaining consistent adherence to 21 CFR Part 820 FDA, diminishing labor-intensive tasks and ensuring error-free records.
Real-world examples illustrate the importance of the QSR in practice. Organizations that have effectively incorporated QSR principles into their operations indicate enhanced control measures and streamlined FDA inspections, ultimately resulting in quicker market access for their products. Furthermore, producers must keep the Quality System Record for a minimum of two years following device transfer, ensuring continued adherence. As the landscape of medical device manufacturing continues to evolve, the QSR and 21 CFR Part 820 FDA remain critical frameworks for ensuring that manufacturers meet the stringent requirements set forth by the FDA.
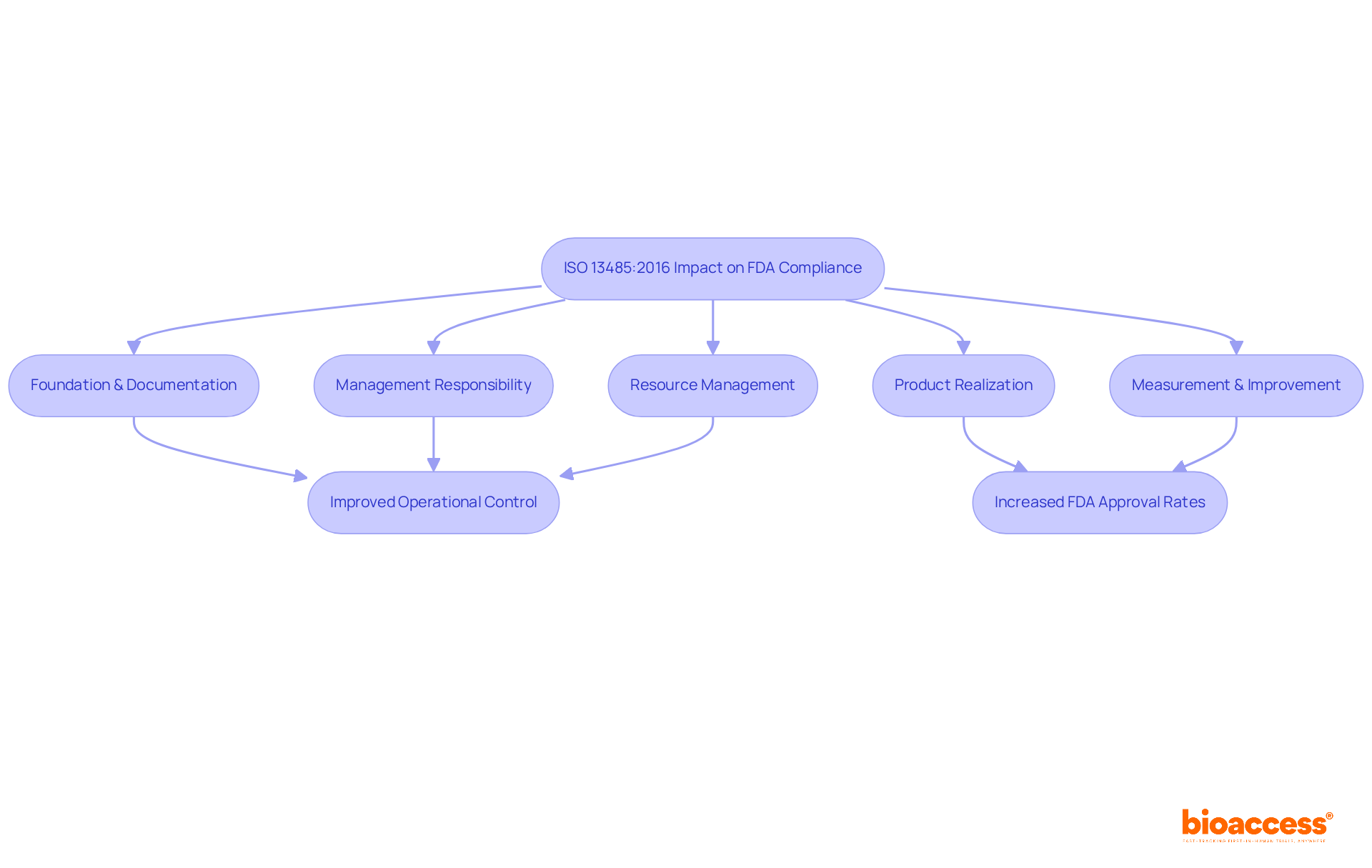
ISO 13485:2016 serves as a vital international standard that delineates the requirements for a management system specifically tailored for the medical device industry. The FDA has acknowledged the significance of this standard, integrating essential components of ISO 13485 into its regulations. As a result, adherence to ISO standards not only facilitates compliance with FDA requirements but also streamlines manufacturing processes and enhances product quality. This alignment markedly enhances manufacturers' chances for successful FDA approval.
The standard is organized around five fundamental clauses:
Organizations that have effectively aligned their management systems with ISO 13485 have reported improved operational control and product safety, which are critical elements in the FDA's evaluation process. The FDA's recent shift towards a Quality Management System Regulation (QMSR) that closely mirrors ISO 13485:2016 underscores the growing importance of 21 CFR Part 820 FDA in achieving regulatory compliance. By early 2026, the U.S. System Regulation known as 21 CFR Part 820 FDA will be officially supplanted by ISO 13485:2016, necessitating manufacturers to adjust their standards accordingly while ensuring compliance with the QSR for a transitional period of two years.
In 2023, ISO 13485 certifications saw a 9% increase, reflecting the industry's movement towards alignment with this standard. Industry leaders assert that conformity with ISO standards not only fulfills regulatory demands but also cultivates a culture of continuous improvement and risk management. As specialists have noted, "Certification indicates that your assurance system is not only operational but designed to withstand regulatory examination." This proactive approach to managing standards can lead to heightened FDA approval rates, as manufacturers demonstrate their commitment to maintaining rigorous standards throughout the product lifecycle.
To prepare for ISO 13485 certification, organizations should undertake internal audits and gap analyses to ensure their management systems are both robust and compliant. Furthermore, clearly defining the scope of the Quality Management System (QMS) is essential for effective audit preparation. In conclusion, the integration of ISO 13485:2016 into FDA compliance strategies transcends mere regulatory obligation; it represents a strategic advantage that elevates product standards, operational efficiency, and ultimately, market access.
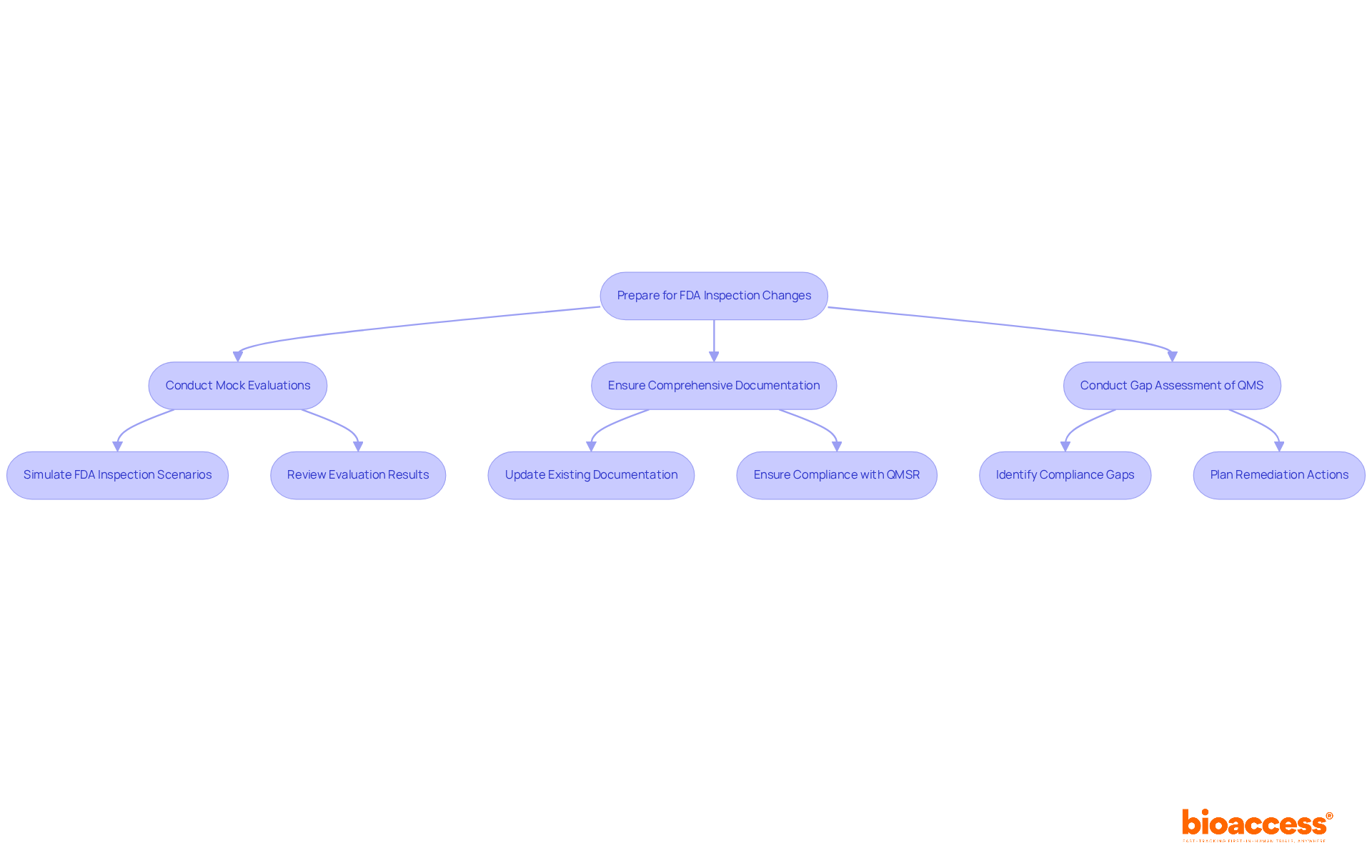
Beginning in February 2026, the FDA will implement new assessment procedures focused on a comprehensive review of quality management systems. Manufacturers must prepare for increased scrutiny, which will include thorough evaluations of management reviews, internal audits, and supplier audits.
To meet these new standards, companies are strongly encouraged to:
The FDA's increased examination frequency—rising from 2.98 to 4.27 warning letters per 100 evaluations between 2019 and 2023, marking a significant 43% increase—underscores the critical need for robust adherence measures. Organizations that proactively prepare for these changes will enhance their operational efficiency and protect their market access in a more regulated environment.
Regulatory experts emphasize that early preparation is essential for maintaining development schedules and ensuring compliance with the evolving landscape. As the FDA transitions from the Quality System Regulation (QSR) to the Quality Management System Regulation (QMSR), manufacturers should conduct a gap assessment of their existing Quality Management Systems to ensure compliance with 21 CFR Part 820 FDA and pinpoint areas requiring improvement.
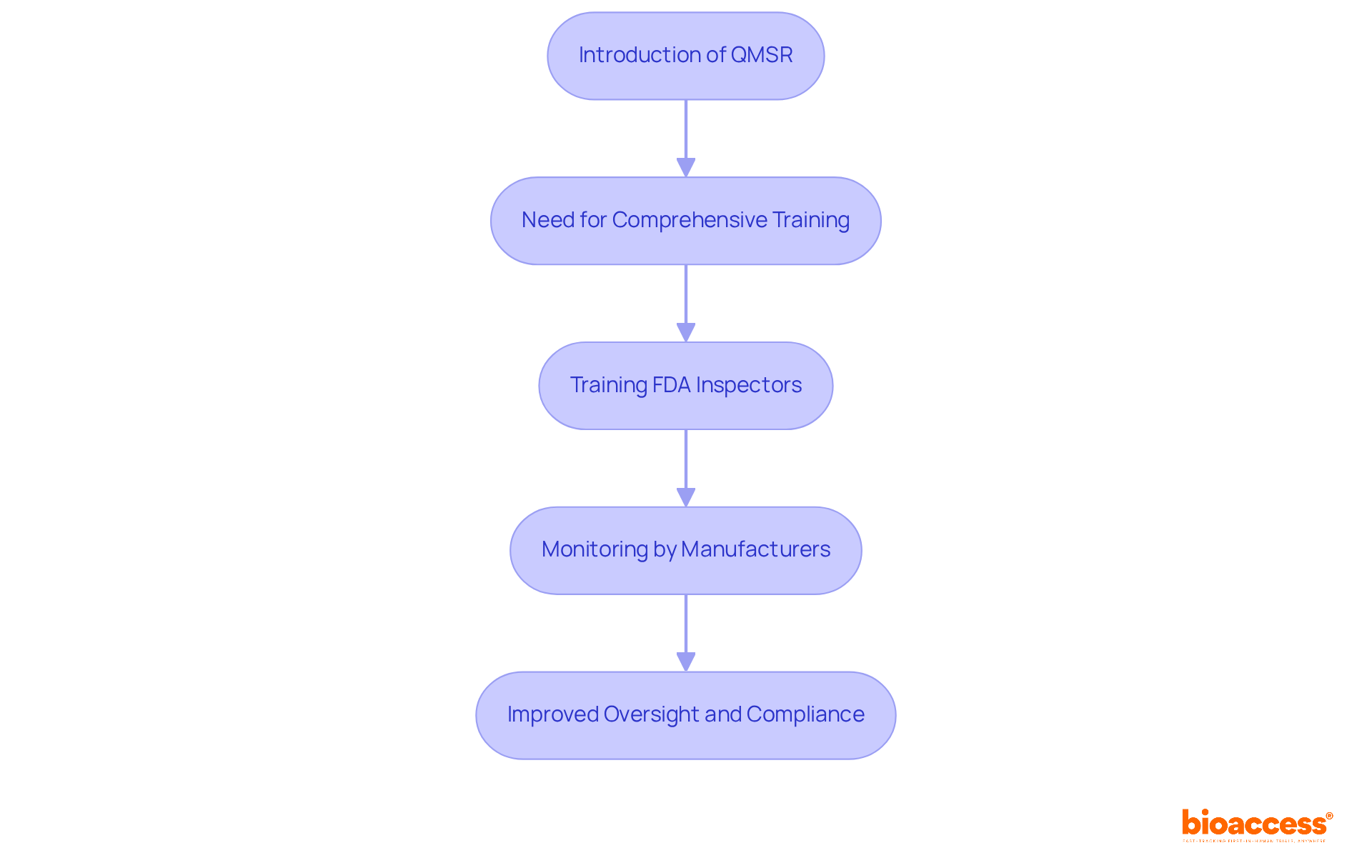
As the FDA gears up for the transition to the new Quality Management System Regulation (QMSR), effective February 2, 2026, comprehensive training for FDA staff on the updated requirements is essential. This training equips inspectors with the vital knowledge needed to evaluate adherence during assessments, thereby enhancing the quality of oversight.
Manufacturers must actively monitor these training initiatives and comprehend how these regulatory changes may affect their interactions with the FDA. By fostering a knowledgeable evaluation workforce, the FDA aims to improve the consistency and effectiveness of its assessments, which is crucial for maintaining high standards in medical device safety and efficacy.
Notably, the QMSR is projected to yield annualized net cost savings of approximately $532 million, underscoring the financial advantages of compliance. Furthermore, the FDA has reaffirmed its commitment to providing guidance and support throughout this transition, recognizing the importance of a well-prepared evaluation team in addressing the anticipated challenges related to investigator attrition.
Beginning February 2026, the FDA will intensify its scrutiny of essential records during inspections, including:
It is imperative for manufacturers to maintain these records in a complete, accurate, and easily accessible manner. A robust documentation system not only simplifies adherence but also demonstrates a strong commitment to quality and safety. Organizations that prioritize Good Documentation Practices (GDocP) are better positioned to withstand the increased scrutiny expected under the new regulations.
Manufacturers that have introduced automated regulatory oversight systems report improved operational efficiency and fewer errors, highlighting the significance of careful documentation. As regulatory specialists emphasize, "In FDA-regulated industries, documentation isn’t merely a formality—it’s the cornerstone of adherence."
This proactive approach to documentation will be crucial as the FDA shifts its focus to evaluating the effectiveness of control measures and risk management practices, ensuring that manufacturers are prepared for the evolving regulatory landscape.
Internal audits and management evaluations are critical elements of a robust management system, particularly in the Medtech sector. These processes empower organizations to identify compliance gaps, assess the effectiveness of their operations, and implement necessary corrective actions. Routine evaluations not only assist manufacturers in adhering to FDA standards but also foster a culture of continuous improvement within their operational systems.
Data indicates that organizations prioritizing continuous improvement practices are better positioned to enhance their systems. For example, companies embracing a quality-first mindset can significantly lower defect rates and boost overall operational efficiency. This proactive strategy facilitates the timely identification of issues and the execution of improvements.
Experts in management standards underscore the indispensable nature of these evaluations. W. Edwards Deming remarked, 'if you can't describe what you are doing as a process, you don't know what you're doing,' underscoring the importance of structured evaluations. Additionally, John Ruskin stated, 'Quality is never an accident. It is consistently the outcome of intelligent effort,' highlighting the value of intentional management practices. Furthermore, the FDA's 2025 updates emphasize that management reviews should be documented and encompass a thorough analysis of audit findings, ensuring organizations not only meet regulatory requirements but also enhance their operational efficiency.
Case studies vividly illustrate the beneficial effects of stringent internal audits. For instance, Toyota's implementation of Deming's principles led to significant improvements in standards and efficiency, allowing the company to outpace its competitors. This commitment to excellence not only ensured compliance but also bolstered the company's market position.
In conclusion, the integration of routine internal audits and management evaluations is essential for Medtech firms striving for excellence in management and ensuring compliance with 21 CFR Part 820 FDA. By prioritizing these practices, organizations can attain elevated standards of quality and operational effectiveness.
To effectively prepare for FDA device evaluations, manufacturers must adopt a multifaceted strategy that encompasses:
A pivotal aspect of this preparation is the execution of mock evaluations, which function as practice runs to pinpoint gaps in readiness and bolster staff confidence. These simulations not only educate employees about evaluation procedures but also aid organizations in identifying areas for enhancement.
In 2025, the emphasis on simulated evaluations is underscored by an anticipated 40% increase in total FDA reviews for FY 2024-25, making proactive adherence efforts essential for companies. Moreover, conducting unexpected mock evaluations and maintaining an 'Evaluation Readiness Kit' can streamline the review process.
Fostering a culture of compliance within the organization ensures that all employees understand the significance of adhering to regulatory requirements, including the importance of training records and compliance with UDI and GUDID requirements. By integrating these strategies, manufacturers can significantly enhance their inspection readiness and position themselves advantageously within the competitive Medtech landscape.
Manufacturers must remain proactive as ISO standards undergo systematic reviews and potential revisions, ensuring that their quality management systems are adaptable. Modifications to these standards can significantly impact compliance with 21 CFR Part 820 FDA, making it essential to monitor updates closely and assess their consistency with current practices.
Engaging with industry organizations and regulatory entities, particularly those led by specialists such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, can provide valuable insights into forthcoming changes and best practices for compliance. Ana's extensive background in biomedical engineering and health economics, along with her consulting experience with global companies, underscores the importance of leveraging expert knowledge to navigate these regulatory landscapes.
To effectively adjust to these evolving standards, producers should prioritize:
FDA evaluations under the new Quality Management System Regulations (QMSR) will differ significantly from Medical Device Single Audit Program (MDSAP) audits in several key aspects. Both processes aim to ensure compliance with quality management standards; however, FDA inspections place a greater emphasis on the effectiveness of the quality system and its implementation. Conversely, MDSAP audits offer a more comprehensive assessment of compliance across multiple jurisdictions. Understanding these distinctions is crucial for manufacturers, as it enables them to tailor their preparation strategies effectively.

The insights presented on 21 CFR Part 820 FDA compliance underscore the critical importance of adhering to regulatory standards in the Medtech industry. Effective compliance not only ensures product safety and efficacy but also enhances operational efficiency and market readiness. With the impending changes in inspection processes and the transition to the Quality Management System Regulation (QMSR), manufacturers must remain vigilant in their preparation and adherence strategies.
Throughout the article, key themes emerge, including:
The proactive approaches discussed, such as conducting mock evaluations and maintaining comprehensive records, are essential for navigating the evolving regulatory landscape. As manufacturers prepare for the increased scrutiny expected in 2026, embracing these strategies will be vital in ensuring compliance and maintaining a competitive edge.
In light of these developments, it is imperative for Medtech leaders to prioritize regulatory adherence as a core component of their operational strategy. By fostering a culture of compliance and investing in training and documentation practices, organizations can not only meet regulatory requirements but also drive innovation and improve patient outcomes. The path to successful FDA compliance is paved with diligence, foresight, and a commitment to quality that ultimately benefits both manufacturers and the patients they serve.
What is bioaccess® and how does it assist Medtech innovators?
bioaccess® accelerates FDA compliance for Medtech innovators by leveraging regulatory agility in Latin America, diverse patient demographics in the Balkans, and efficient routes in Australia. It enables ethical approvals in 4-6 weeks and faster enrollment processes, enhancing the ability to bring innovations to market quickly.
Why is regulatory speed important for Medtech companies?
Regulatory speed is vital for maintaining a competitive edge, allowing companies to respond swiftly to market needs and seize emerging opportunities. It helps Medtech firms meet the growing demand for quicker evaluations in the sector.
What strategies can Medtech firms implement to ensure FDA compliance?
Effective adherence strategies include meticulous documentation and proactive engagement with regulatory bodies, which help navigate the complexities of the FDA submission process while ensuring compliance with 21 CFR Part 820.
What is the Quality System Regulation (QSR) under 21 CFR Part 820?
The QSR is a set of requirements that medical device manufacturers must implement as part of a robust Quality Management System (QMS). It covers aspects such as design, manufacturing, packaging, labeling, storage, installation, and servicing of medical devices.
What are some common compliance deficiencies noted by the FDA?
Common deficiencies include issues with design controls, CAPA (Corrective and Preventive Actions) deficiencies, and problems related to 'Complaint Files,' as evidenced by the Warning Letters issued by the FDA.
How can manufacturers improve compliance with the QSR?
Manufacturers can improve compliance by creating thorough documentation, implementing strict control procedures, and utilizing automated document control systems to minimize human error and ensure consistent adherence to regulations.
What role does ISO 13485:2016 play in FDA compliance?
ISO 13485:2016 is an international standard that outlines requirements for a management system in the medical device industry. Compliance with this standard facilitates FDA compliance, streamlines manufacturing processes, and enhances product quality.
What changes are expected regarding the QSR and ISO 13485:2016 by early 2026?
By early 2026, the U.S. System Regulation known as 21 CFR Part 820 will be replaced by ISO 13485:2016, requiring manufacturers to adjust their standards while ensuring compliance with the QSR during a transitional period of two years.
How can organizations prepare for ISO 13485 certification?
Organizations should conduct internal audits and gap analyses to ensure their management systems are robust and compliant. Clearly defining the scope of their Quality Management System (QMS) is also essential for effective audit preparation.
What benefits do organizations gain from aligning with ISO 13485?
Aligning with ISO 13485 leads to improved operational control, enhanced product safety, and increased chances of successful FDA approval, fostering a culture of continuous improvement and risk management.