


The article emphasizes the critical types of clinical research monitoring that are pivotal for achieving successful outcomes in medical studies. It delineates various monitoring approaches, including:
Each method is discussed in terms of its contribution to enhancing data integrity, ensuring compliance, and improving overall efficiency in clinical trials. This focus ultimately supports the advancement of Medtech innovations.
In the rapidly evolving landscape of medical technology, effective clinical research monitoring stands as a cornerstone for success. This critical function not only enhances patient access to innovative therapies but also significantly improves healthcare outcomes. For stakeholders in the Medtech industry, understanding the various types of monitoring methods is crucial.
However, as the demand for efficiency and compliance intensifies, organizations must ask themselves: how can they ensure they are leveraging the right strategies to navigate the complexities of clinical trials?
This article explores nine essential types of clinical research monitoring, highlighting their unique benefits and the pivotal role they play in advancing medical innovations.
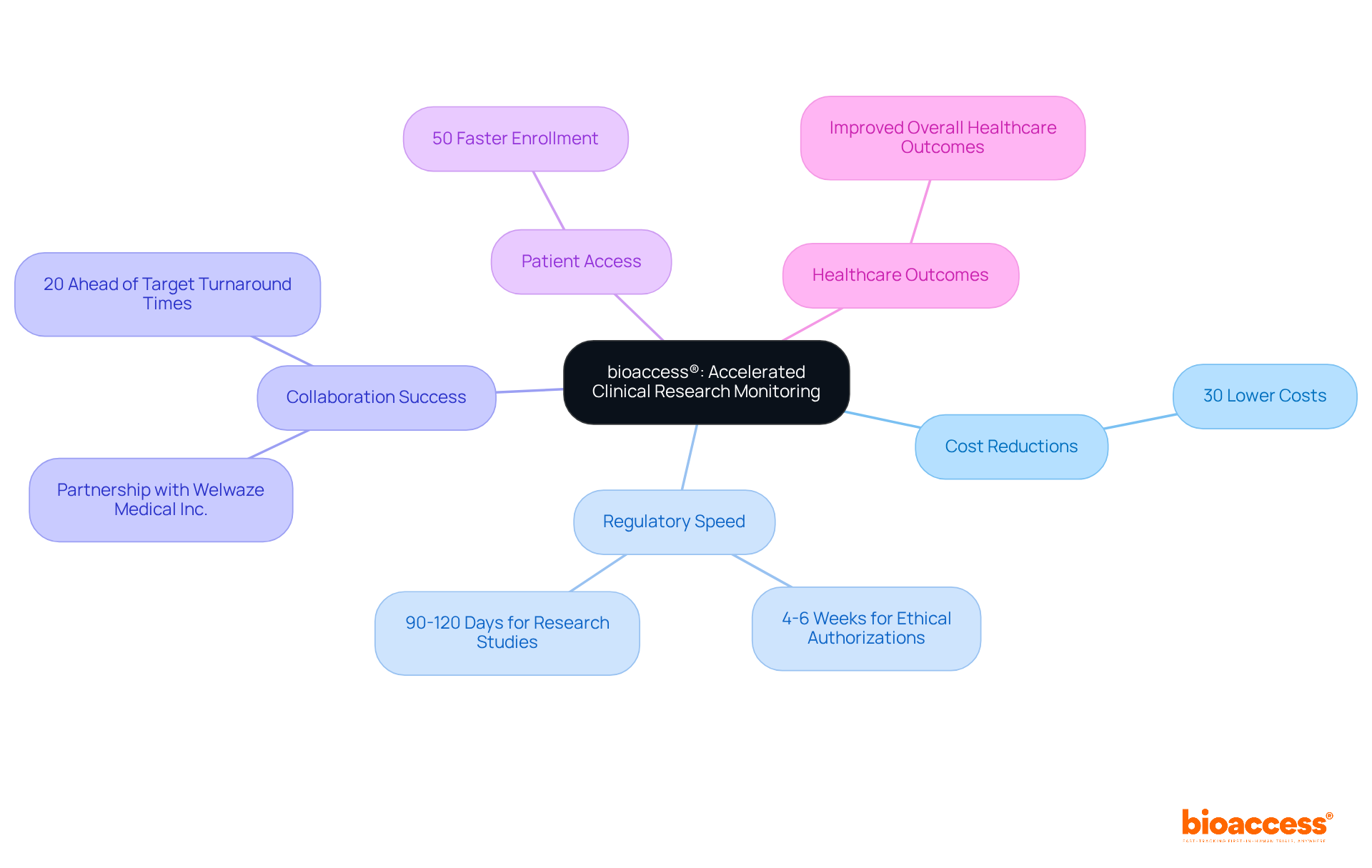
bioaccess® distinguishes itself in the research field through its clinical research monitoring services, which are expedited and specifically designed for Medtech innovations. By leveraging Colombia's competitive advantages—such as cost reductions exceeding 30% compared to North America and Western Europe, rapid regulatory processes that enable ethical authorizations in just 4-6 weeks, and a globally recognized top-tier healthcare system—bioaccess® ensures that studies are not only compliant but also remarkably efficient.
This strategic approach allows Medtech companies to accelerate their innovations to market, significantly enhancing patient access to new therapies and improving overall healthcare outcomes. For instance, research studies conducted in Colombia can secure ethical approvals in merely 90-120 days, facilitating enrollment that is 50% faster than in traditional markets. Such efficiencies highlight the vital role of regulatory speed in advancing medical technologies, ultimately benefiting both innovators and the healthcare systems they support.
Notably, bioaccess® has successfully collaborated with Welwaze Medical Inc. for the launch of the Celbrea® medical device, showcasing the effectiveness of their evaluation services. Furthermore, their average full-board IRB determinations arrive 20% ahead of targeted turnaround times, expediting site activation.
As Scott Whitaker, President and CEO of AdvaMed, emphasizes, 'The significance of regulatory speed in medical studies cannot be overstated; it is crucial for ensuring that innovative therapies reach patients promptly.'
Collectively, these elements underscore bioaccess®'s commitment to clinical research monitoring and its positive impact on enhancing research oversight and Medtech innovations.
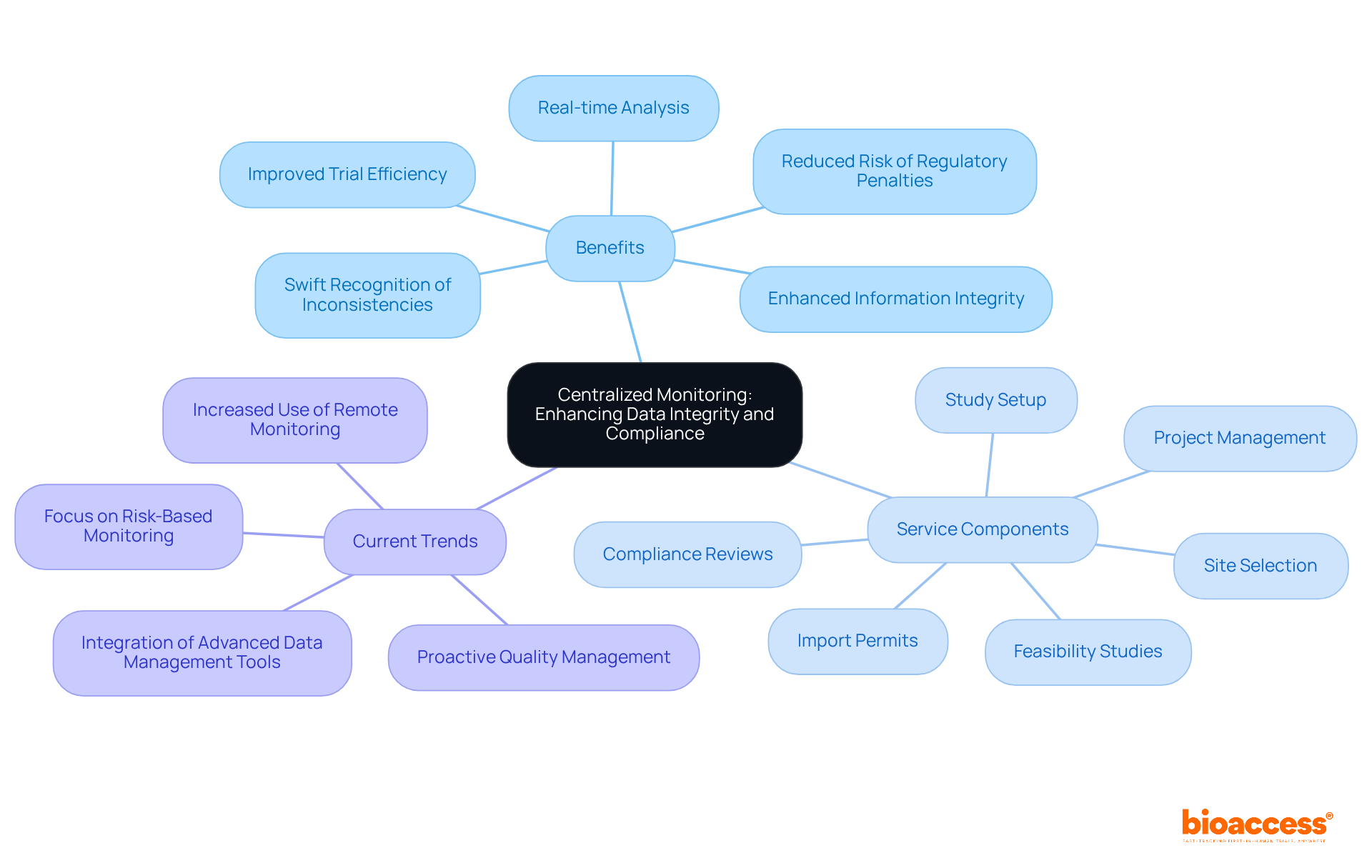
Centralized clinical research monitoring serves as a pivotal tool in simplifying the supervision of research information from a singular site. This approach not only enables real-time analysis but also facilitates the swift recognition of inconsistencies. By ensuring uniform adherence to protocols and standards across all sites, it significantly enhances information integrity. Leveraging sophisticated information management systems allows research teams to swiftly address compliance challenges, thereby reducing the risk of regulatory penalties and bolstering the dependability of study results.
bioaccess's comprehensive clinical study management services encompass:
Each of these elements contributes to advancing global health improvement through innovation in Medtech. Current trends indicate that clinical research monitoring through centralized oversight not only boosts compliance rates but also fosters a proactive atmosphere for quality management. Experts in the field advocate for this method in clinical research monitoring, highlighting its effectiveness in identifying systematic errors and enhancing overall trial efficiency.
For instance, centralized oversight has proven instrumental in revealing significant issues related to information integrity, even concerning non-critical data. This capability facilitates clinical research monitoring by enabling targeted interventions at higher-risk locations, which ultimately leads to improved information quality and more reliable research outcomes in healthcare. Embracing this strategic method is essential for those committed to excellence in clinical research monitoring.
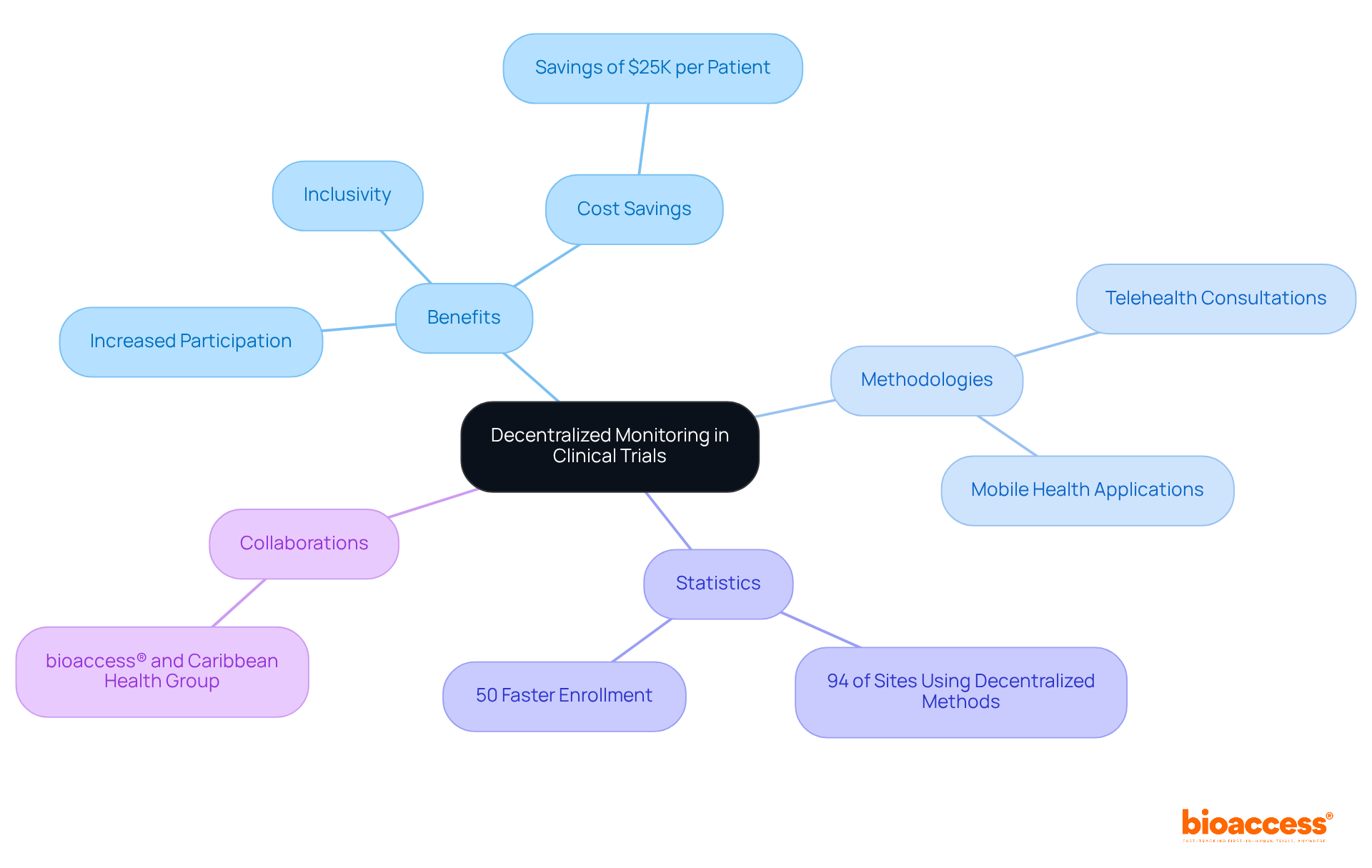
Decentralized monitoring offers a versatile framework for clinical research monitoring, empowering researchers to collect data from diverse locations without requiring participants to travel to a central site. This innovative approach significantly boosts patient engagement, demonstrated by a remarkable increase in participation rates. A survey revealed that 94% of research sites incorporated at least one decentralized methodology in 2021, underscoring the growing acceptance of this model.
By leveraging digital tools and remote data collection techniques, such as telehealth consultations and mobile health applications, researchers can effectively manage studies while addressing participants' needs. This leads to a more diverse and representative participant pool. Such adaptability not only fosters inclusivity but also yields more substantial results, ultimately driving the advancement of innovative medical solutions.
Additionally, bioaccess® has proven its ability to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, achieving significant cost savings of $25K per patient with FDA-ready data—no rework, no delays. This efficiency is further bolstered by the collaboration between bioaccess™ and Caribbean Health Group, which aims to position Barranquilla as a key hub for medical studies in Latin America, supported by Colombia's Minister of Health.
As Hana Lee from the FDA emphasizes, decentralized studies enhance participation and facilitate continuous data collection in real-world settings, which is essential for clinical research monitoring and highlights their potential impact.
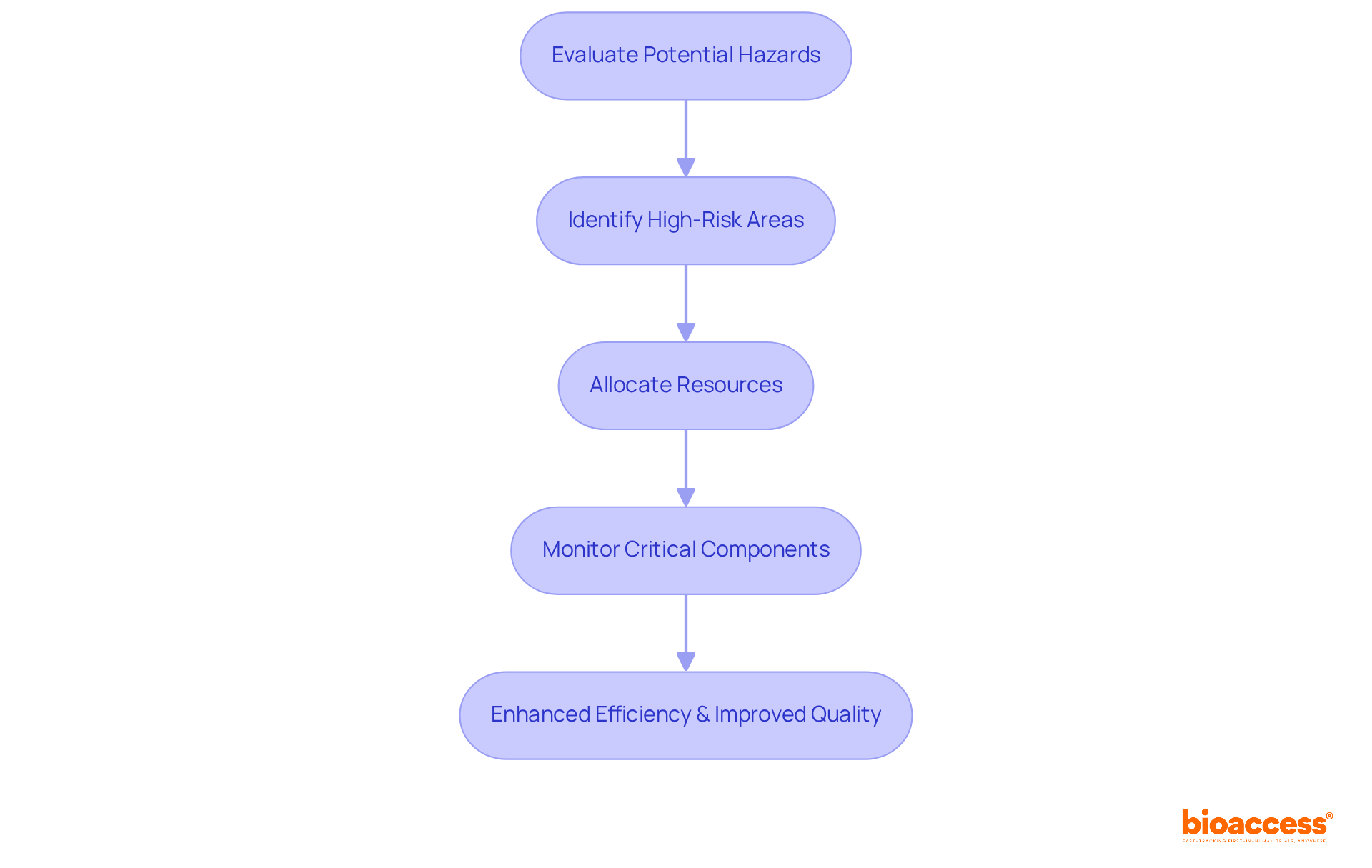
Risk-based monitoring (RBM) serves as a strategic approach in clinical research monitoring that evaluates potential hazards in clinical studies, prioritizing monitoring activities accordingly. By concentrating on high-risk areas, researchers can allocate resources more effectively, ensuring that critical components of the study receive necessary oversight. This targeted approach not only enhances efficiency but also significantly improves the overall quality of the evaluation by proactively addressing potential issues before they escalate.
Recent findings indicate that RBM has led to a remarkable 28% reduction in significant findings per clinical quality control visit, underscoring its effectiveness in enhancing study oversight. Additionally, the methodology has achieved a 29.3% decrease in median query resolution time, reflecting improved operational efficiency.
Current trends in resource allocation for RBM underscore the importance of adaptive oversight, where resources are reallocated from well-performing sites to those requiring additional attention. This dynamic approach fosters better management of testing resources, ultimately enhancing patient safety and ensuring data integrity.
Industry leaders emphasize the critical nature of this proactive resource allocation. As one expert noted, 'By pinpointing high-risk areas, resources can be distributed effectively to guarantee adequate supervision and oversight.' This perspective reinforces the significance of clinical research monitoring in optimizing study management and enhancing the likelihood of favorable outcomes.
Remote supervision leverages advanced technology to facilitate clinical research monitoring, delivering real-time oversight of clinical trials and empowering researchers to monitor participant progress and gather information from virtually any location. This capability significantly enhances participant safety by enabling immediate responses to adverse events or data anomalies.
Research indicates that remote observation can lead to:
This underscores its impact on patient outcomes. By integrating distant observation tools, research teams can improve their responsiveness and uphold high standards of care throughout the study process.
Furthermore, the utilization of real-time information allows Clinical Research Organizations (CROs) to swiftly adjust strategies in clinical research monitoring, ensuring optimal site performance and enhancing overall study efficiency. As the industry increasingly adopts remote oversight, the advantages for participant safety and information integrity become increasingly apparent, establishing it as an essential component of contemporary research.
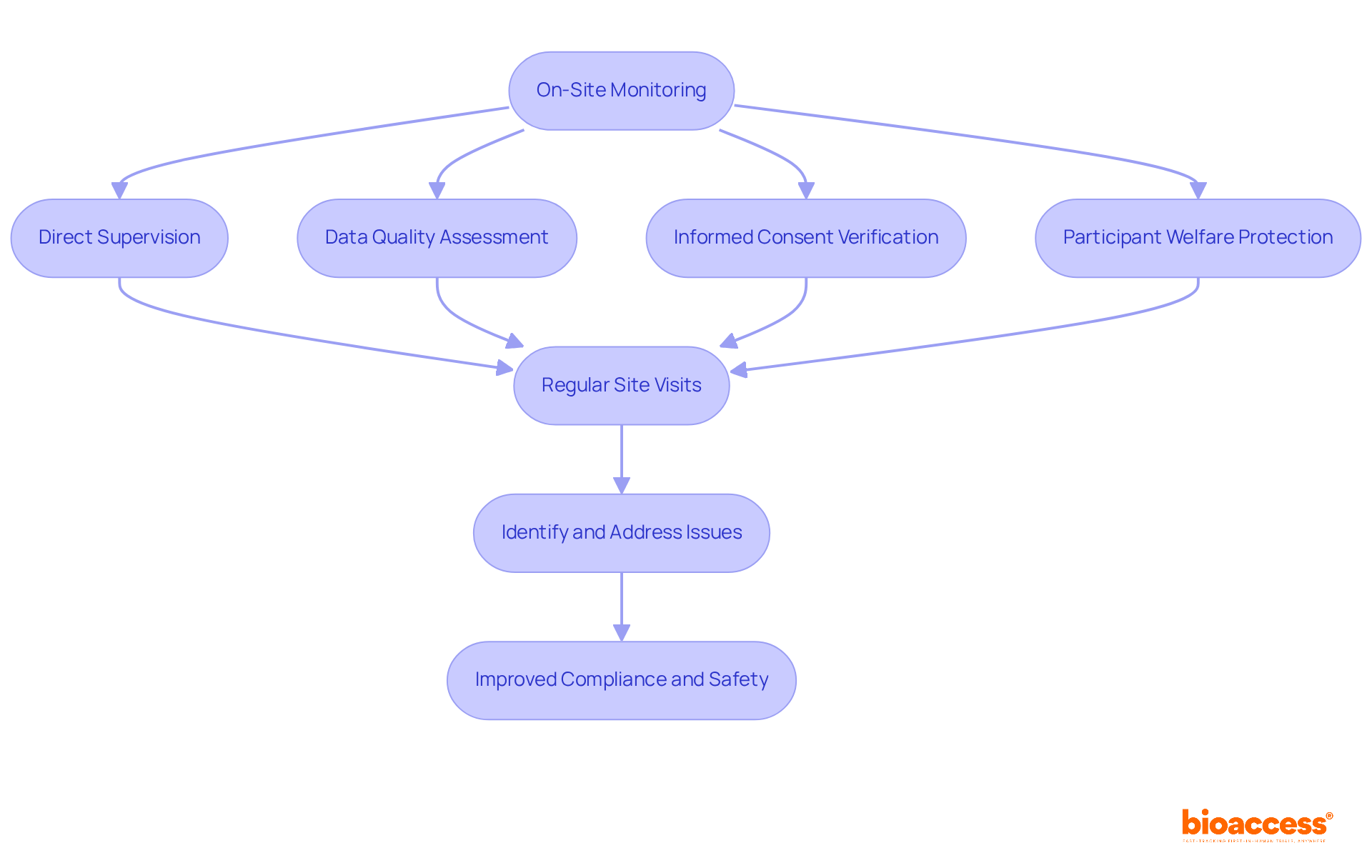
Clinical research monitoring through on-site oversight is essential for ensuring adherence and safety in medical studies, involving direct supervision of activities at the research site. This method allows monitors to assess the quality of data collection, verify informed consent processes, and protect participant welfare.
Regular site visits are essential for clinical research monitoring, enabling research teams to swiftly identify and address issues, thereby preserving the integrity of trials. Recent studies indicate that on-site supervision significantly improves ethical conduct, with a remarkable 96.42% enhancement in compliance following active oversight interventions.
Furthermore, a retrospective analysis of 22 oversight reports uncovered 56 deviations, primarily concerning documentation and record-keeping, highlighting the necessity of thorough supervision. By addressing these deviations, oversight teams not only ensured adherence to protocols but also reinforced participant safety, illustrating the critical role of on-site supervision in research.
At bioaccess, our comprehensive clinical study management services encompass:
All designed to promote innovation and regulatory excellence in Medtech clinical research throughout Latin America.
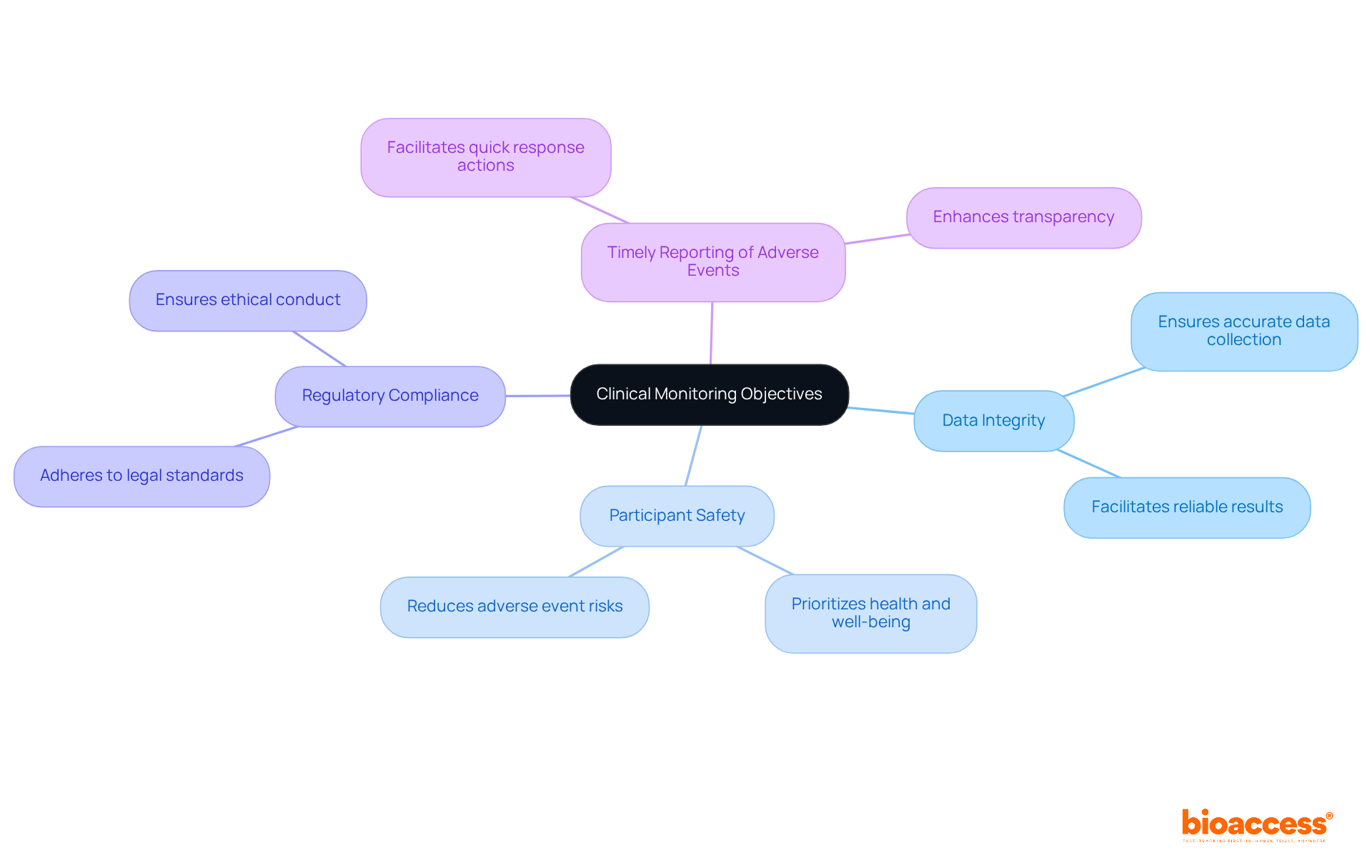
The objectives of clinical research monitoring serve as a strategic roadmap for study management, guiding the research team's activities and ensuring alignment with overarching study goals. Essential objectives encompass:
By establishing these objectives, clinical research monitoring teams can concentrate on the study's most vital elements, significantly increasing the chances of success.
The application of Risk-Based Quality Management (RBQM) in clinical research monitoring has shown that studies with clearly defined goals experience higher adoption rates of oversight practices. In 2021, 88% of studies included at least one RBQM element, indicating a rising awareness of the significance of clinical research monitoring. Furthermore, the projected completion rates for Phase III studies hit 84.9% in 2018, highlighting the influence of clinical research monitoring on results.
As Albert Einstein wisely remarked, 'If we knew what we were doing, it would not be called research,' emphasizing the importance of clear goals to manage the uncertainties inherent in medical studies. By prioritizing clearly defined evaluation goals, clinical research monitoring can help research teams improve their focus and achieve successful study outcomes.
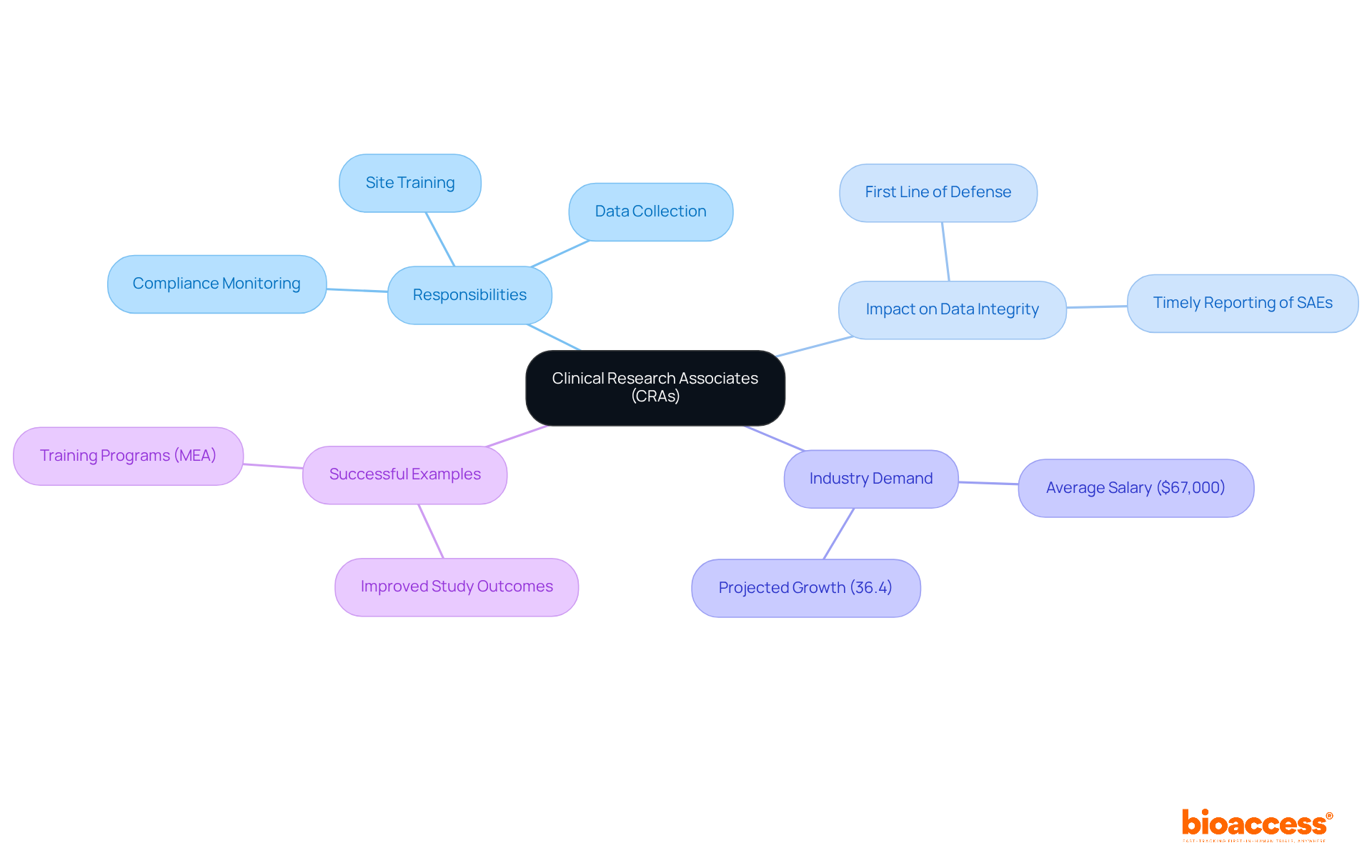
Clinical Research Associates (CRAs) play a pivotal role in the monitoring process, serving as the primary link between research sites and sponsors. Their responsibilities encompass ensuring compliance with regulatory standards and the accurate collection of data, which is vital for maintaining the integrity of clinical studies. CRAs conduct regular site visits, meticulously review documentation, and provide essential training to site staff, thereby upholding the quality of the study.
The influence of CRAs on data integrity is profound. They often serve as the first line of defense against compliance errors, ensuring that every aspect of the study aligns with established protocols. For example, delays in reporting serious adverse events (SAEs) can result in significant compliance challenges, underscoring the necessity of timely and precise communication from CRAs to the Principal Investigator (PI) and the sponsor.
Recent trends reveal an increasing demand for qualified CRAs, with projections indicating a 36.4% rise in demand from 2012 to 2022 in the U.S. This surge underscores the critical need for skilled professionals capable of navigating the complexities of clinical studies. Organizations such as Covance have acknowledged this requirement and have initiated comprehensive training programs, including the Monitoring Excellence Academy (MEA), to enhance the competencies of CRAs and prepare them for industry challenges.
Industry leaders highlight the significance of CRAs in ensuring compliance and data integrity. As one expert remarked, "Good TMF management clearly sets up what is to be expected as far as getting documents from both a country level and a site level." This statement emphasizes the need for CRAs to establish effective communication channels and maintain rigorous documentation practices.
Numerous successful examples illustrate CRAs' commitment to ensuring adherence, with many organizations reporting improved study outcomes attributed to the diligent efforts of their oversight teams. By fostering strong relationships with site personnel and adopting a proactive approach to compliance, CRAs occupy a vital role in the success of research studies, ultimately contributing to advancements in medical technology and patient care.
Compliance considerations are paramount in clinical research monitoring, as they ensure adherence to regulatory standards and ethical guidelines. Key elements of clinical research monitoring encompass:
At bioaccess, our extensive clinical research monitoring services encompass:
Each is critical for upholding high compliance levels. Prioritizing compliance in clinical research monitoring not only safeguards participant welfare but also enhances the study's integrity, leading to more reliable and valid outcomes. For instance, research indicates that overall compliance rates were 71% ± 31% during the initial 13 weeks, highlighting the necessity of maintaining elevated compliance levels to support the internal validity of trials.
Furthermore, effective oversight practices, particularly clinical research monitoring using electronic data capture (EDC) systems, have demonstrated improvements in data integrity and compliance rates, with nearly 80% of research studies adopting these technologies. Regulatory specialists emphasize that establishing explicit healthcare observation goals and adherence criteria is vital for ensuring patient safety and collecting high-quality data. By implementing targeted oversight plans and fostering a culture of compliance, clinical research monitoring can significantly bolster the credibility of research teams' study outcomes.
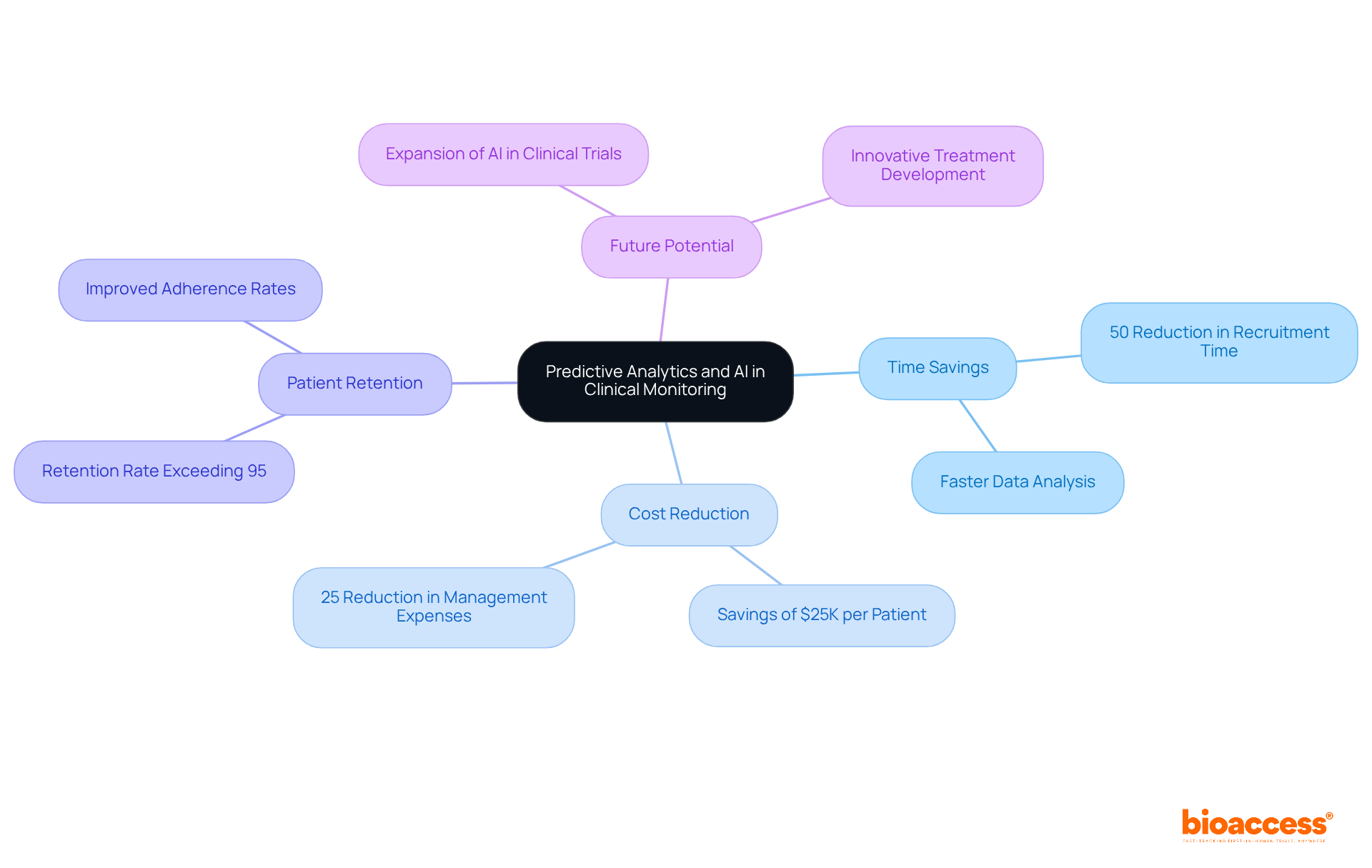
Predictive analytics and artificial intelligence (AI) are fundamentally transforming patient monitoring, facilitating advanced data analysis and informed decision-making. These technologies enable the instantaneous recognition of trends and potential issues, allowing researchers to proactively address challenges before they adversely affect study results. For instance, bioaccess®'s AI-driven patient recruitment algorithms can reduce recruitment time by over 50%, achieving significant savings of $25K per patient by analyzing large datasets to identify suitable candidates. Moreover, the incorporation of AI in clinical studies has led to a notable decrease in operational costs, with clients experiencing a 25% reduction in management expenses.
Successful examples of AI-driven insights enhancing study outcomes include the collaboration between GlobalCare Clinical Trials and bioaccess™, which resulted in an impressive subject retention rate exceeding 95% and a reduction in recruitment time by more than 50%. This partnership illustrates how predictive analytics can refine patient selection, thereby boosting adherence rates and overall study success. As the healthcare industry increasingly embraces digital transformation, the role of AI in clinical trials is anticipated to expand, fostering more efficient monitoring strategies and ultimately leading to improved patient outcomes. The potential benefits are substantial, paving the way for innovative treatments and enhanced healthcare delivery.
The landscape of clinical research monitoring is rapidly evolving, marked by the emergence of diverse methodologies aimed at enhancing efficiency and compliance within medical studies. This article has examined nine essential types of clinical research monitoring, each playing a crucial role in facilitating the swift and safe introduction of innovations in Medtech to the market. By grasping these approaches, stakeholders can adeptly navigate the complexities of clinical trials, fostering advancements that ultimately enhance patient care.
Key insights reveal the advantages of both centralized and decentralized monitoring, each offering distinct benefits in terms of data integrity and participant engagement, respectively. Risk-based monitoring stands out as a strategic method for effective resource allocation, while remote monitoring showcases the potential for real-time oversight, significantly bolstering patient safety. On-site monitoring remains indispensable for compliance, with the role of Clinical Research Associates (CRAs) highlighted as vital for maintaining data integrity. Furthermore, the integration of predictive analytics and AI is revolutionizing the analysis and utilization of data in clinical trials, paving the way for more informed decision-making.
As the healthcare industry increasingly embraces these innovative monitoring strategies, it is imperative for researchers and organizations to adopt best practices that prioritize compliance, efficiency, and patient safety. By leveraging the insights and methodologies discussed, stakeholders can actively contribute to the advancement of clinical research, ensuring that groundbreaking therapies reach patients in a timely manner, ultimately transforming healthcare outcomes for the better.
What is bioaccess® and what services does it provide?
bioaccess® specializes in clinical research monitoring services specifically designed for Medtech innovations, offering expedited processes that ensure compliance and efficiency in research studies.
What advantages does Colombia offer for clinical research through bioaccess®?
Colombia provides cost reductions exceeding 30% compared to North America and Western Europe, rapid regulatory processes with ethical authorizations in 4-6 weeks, and a top-tier healthcare system recognized globally.
How does bioaccess® enhance the speed of clinical studies?
Research studies conducted in Colombia can secure ethical approvals in 90-120 days, with enrollment speeds 50% faster than traditional markets, highlighting the importance of regulatory speed in advancing medical technologies.
Can you provide an example of bioaccess®'s successful collaboration?
bioaccess® successfully collaborated with Welwaze Medical Inc. for the launch of the Celbrea® medical device, demonstrating the effectiveness of their evaluation services.
What is centralized clinical research monitoring and its benefits?
Centralized clinical research monitoring simplifies the supervision of research data from a single site, enabling real-time analysis and swift recognition of inconsistencies, which enhances data integrity and compliance.
What services are included in bioaccess's clinical study management?
bioaccess's services include feasibility studies, site selection, compliance reviews, study setup, import permits, and project management.
How does decentralized monitoring differ from traditional methods?
Decentralized monitoring allows researchers to collect data from various locations without requiring participants to travel to a central site, significantly increasing patient engagement and participation rates.
What technologies are utilized in decentralized monitoring?
Digital tools such as telehealth consultations and mobile health applications are used for remote data collection, enhancing the management of studies while addressing participants' needs.
What impact does bioaccess® have on patient enrollment and cost savings?
bioaccess® can enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, achieving significant cost savings of $25K per patient with FDA-ready data.
What is the significance of decentralized studies according to experts?
Decentralized studies enhance participant engagement and facilitate continuous data collection in real-world settings, which is crucial for effective clinical research monitoring.