


The article highlights innovative clinical trial designs that are pivotal in accelerating research processes and enhancing efficiency in medical studies. By employing strategies such as:
it demonstrates how these designs facilitate quicker patient recruitment, reduce costs, and improve the overall participant experience. This ultimately leads to expedited approvals and market access for new therapies, underscoring the importance of these advancements in the clinical research landscape.
Innovative clinical trial designs are reshaping the landscape of medical research, offering new pathways to accelerate the development of groundbreaking therapies. By embracing adaptive, decentralized, and pragmatic approaches, researchers can enhance patient engagement, streamline data collection, and ultimately reduce the time and costs associated with traditional trials.
However, as these designs gain traction, a critical question arises: How can stakeholders effectively navigate the complexities of these innovative methodologies to maximize their potential and ensure successful outcomes?
bioaccess® is at the forefront of clinical research, utilizing groundbreaking clinical trial designs that significantly simplify research processes. This innovation leads to substantial reductions in the time and resources required for studies. By employing adaptive, decentralized, and seamless methodologies, bioaccess® enhances the efficiency of clinical trial designs, which ensures rapid patient recruitment and efficient data collection. As a result, this strategic approach facilitates expedited approvals and faster market access for revolutionary medical technologies and therapies.
Decentralized studies exemplify this shift, effectively addressing travel challenges for participants. Notably, 44% of potential participants find travel to study clinics difficult, highlighting the importance of enhancing overall engagement. Furthermore, the integration of advanced technologies, such as electronic data capture systems, is projected to grow at a remarkable compound annual growth rate of 14.6% from 2023 to 2030. This underscores the shift towards more efficient research practices, particularly in clinical trial designs, that bioaccess® champions.
By emphasizing creative concepts, bioaccess® not only accelerates medical studies but also enriches the overall participant experience. This commitment leads to increased retention rates and successful outcomes, reinforcing the necessity of collaboration in advancing clinical research. The future of Medtech depends on innovative solutions like those offered by bioaccess®, prompting stakeholders to consider their own challenges and embrace transformative strategies.
Basket clinical trial designs serve a crucial role in evaluating the effectiveness of diverse treatments targeting a single condition within a cohesive research framework. This innovative design, as seen in clinical trial designs, is particularly beneficial in oncology, where various tumor types may respond similarly to identical treatments. By grouping individuals with different tumor types, researchers can efficiently gather data, significantly reducing both the time and costs typically associated with conventional clinical trial designs. This approach not only accelerates the development of new therapies but also enhances the understanding of treatment effects across diverse patient populations by utilizing effective clinical trial designs.
Notably, the Basket of Baskets (BoB) study has successfully treated over 170 individuals, demonstrating the effectiveness of clinical trial designs in providing personalized therapy options. Furthermore, the implementation of clinical trial designs, including basket studies, can lead to a 20-30% reduction in total research expenses, making them an appealing choice for Medtech firms navigating the complexities of research procedures.
In Colombia, the partnership between bioaccess™ and Caribbean Health Group, announced on March 29, 2019, aims to establish Barranquilla as a key location for medical studies in Latin America, supported by the Minister of Health. This initiative enhances ambulatory services for research, encompassing feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, while also fostering local economic growth through job creation and improved healthcare outcomes.
Clinical trial designs, particularly adaptive study frameworks, empower investigators to make real-time modifications to protocols based on interim data, thereby enhancing the efficiency of clinical research. This flexibility encompasses:
This results in more ethical studies where individuals are less likely to endure ineffective therapies. With bioaccess®'s capability to enroll treatment-naive cardiology or neurology groups 50% faster than Western locations, alongside a realization of $25K savings per patient with FDA-ready data, adaptive approaches significantly reduce the time required to achieve definitive results. This synergy accelerates the path to market for new treatments, rendering bioaccess®'s expert services invaluable for Medtech, Biopharma, and Radiopharma startups.
Clinical trial designs, such as platform studies, represent innovative frameworks that facilitate the simultaneous evaluation of various therapies for a single illness, utilizing a common control group. This methodology significantly enhances efficiency within clinical trial designs, enabling rapid assessment of new therapies as they arise.
By integrating diverse treatment approaches into one of the clinical trial designs, researchers can effectively gather comparative data, leading to quicker conclusions about optimal therapeutic options. This strategy is particularly advantageous in dynamic fields such as oncology, where clinical trial designs prioritize timely results.
For example, successful platform studies like the RECOVERY study have illustrated the capacity to swiftly evaluate multiple therapies, highlighting the versatility of adaptive designs in tackling public health challenges. Furthermore, the effectiveness of clinical trial designs, particularly platform studies, is underscored by their ability to assess several therapies concurrently, which not only accelerates the research process but also improves outcomes for patients.
In Argentina, bioaccess® ensures that enrollment occurs 50% faster than in traditional markets, achieving substantial cost savings of $25K per individual with FDA-ready data—no rework, no delays. The implementation of these effective clinical trial designs has fostered collaboration between researchers and sponsors, streamlining participant recruitment and enhancing data collection.
Additionally, bioaccess® specializes in Accelerated Medical Device Clinical Study Services, providing comprehensive support throughout the study process. As the research landscape evolves, clinical trial designs, particularly platform studies, emerge as a vital approach for advancing medical science and delivering effective therapies to patients.
Choosing bioaccess® for your research endeavors not only expedites the process but also improves cost efficiency, ensuring successful outcomes.
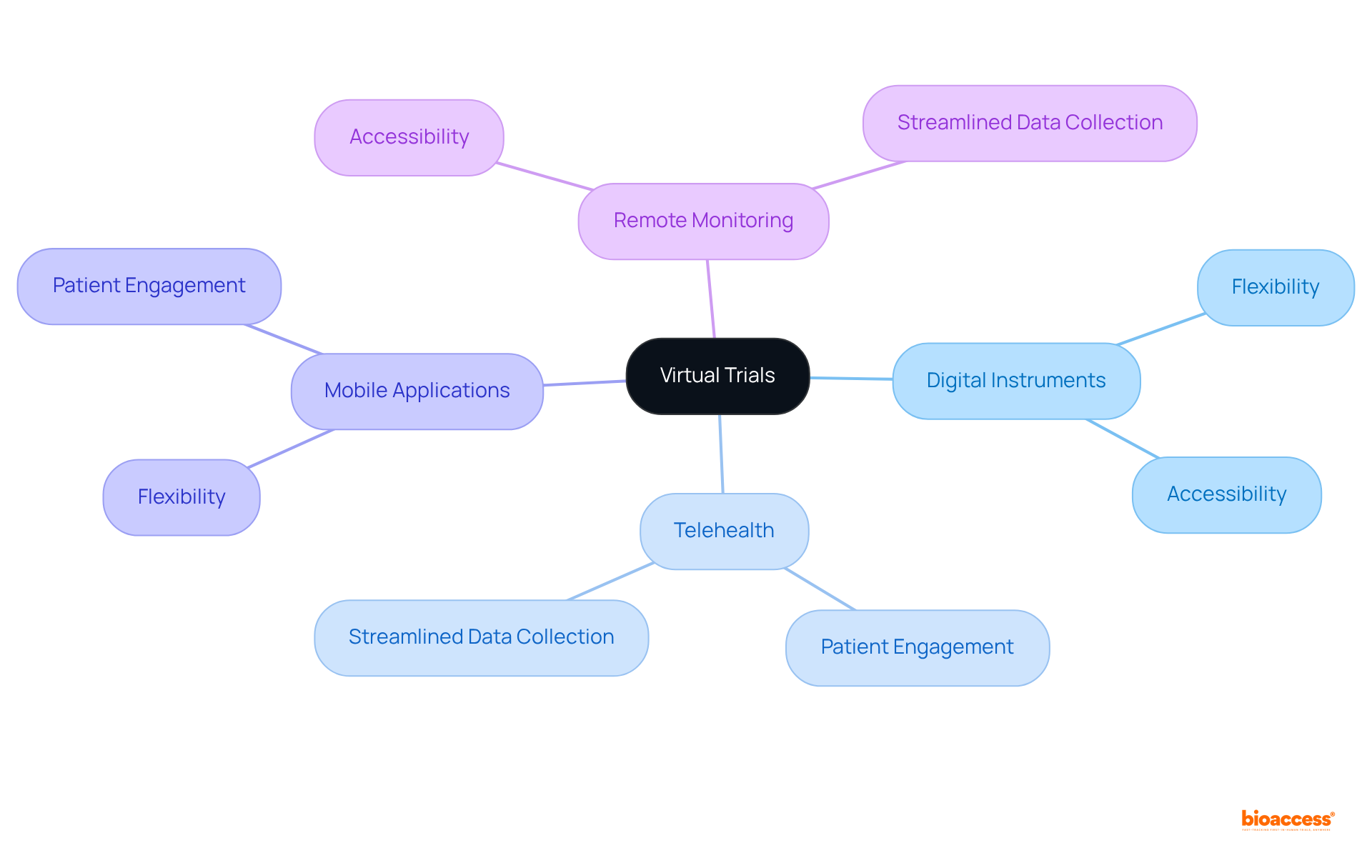
Decentralized clinical studies (DCTs) leverage digital technologies to conduct research remotely, allowing individuals to participate from their homes. This innovative design significantly enhances patient recruitment and retention by alleviating the burdens associated with travel and in-person visits.
By utilizing telemedicine, mobile health applications, and remote monitoring devices, DCTs can gather critical data, ensuring that studies continue even in challenging circumstances, such as during a pandemic. The COVID-19 pandemic has accelerated the integration of decentralized elements, prompting regulators to recognize the potential of DCTs to improve accessibility and diversity in research studies.
Research indicates that DCTs can attract participants who might otherwise be excluded from traditional studies, particularly those facing mobility challenges or residing in remote areas. Additionally, the incorporation of technology into recruitment strategies has proven effective, with digital outreach methods demonstrating higher engagement rates compared to conventional approaches.
As clinical study leaders emphasize, the transition towards DCTs signifies a transformative shift in research methodologies, underscoring the importance of participant-centered designs that prioritize accessibility and inclusivity. However, it remains crucial to foster strong relationships between investigators and participants to ensure effective recruitment and retention.
Furthermore, the implementation of hybrid studies, which combine both decentralized and on-site elements, is proposed as a solution to address some of the challenges faced by DCTs, ensuring robust oversight and active participant involvement.
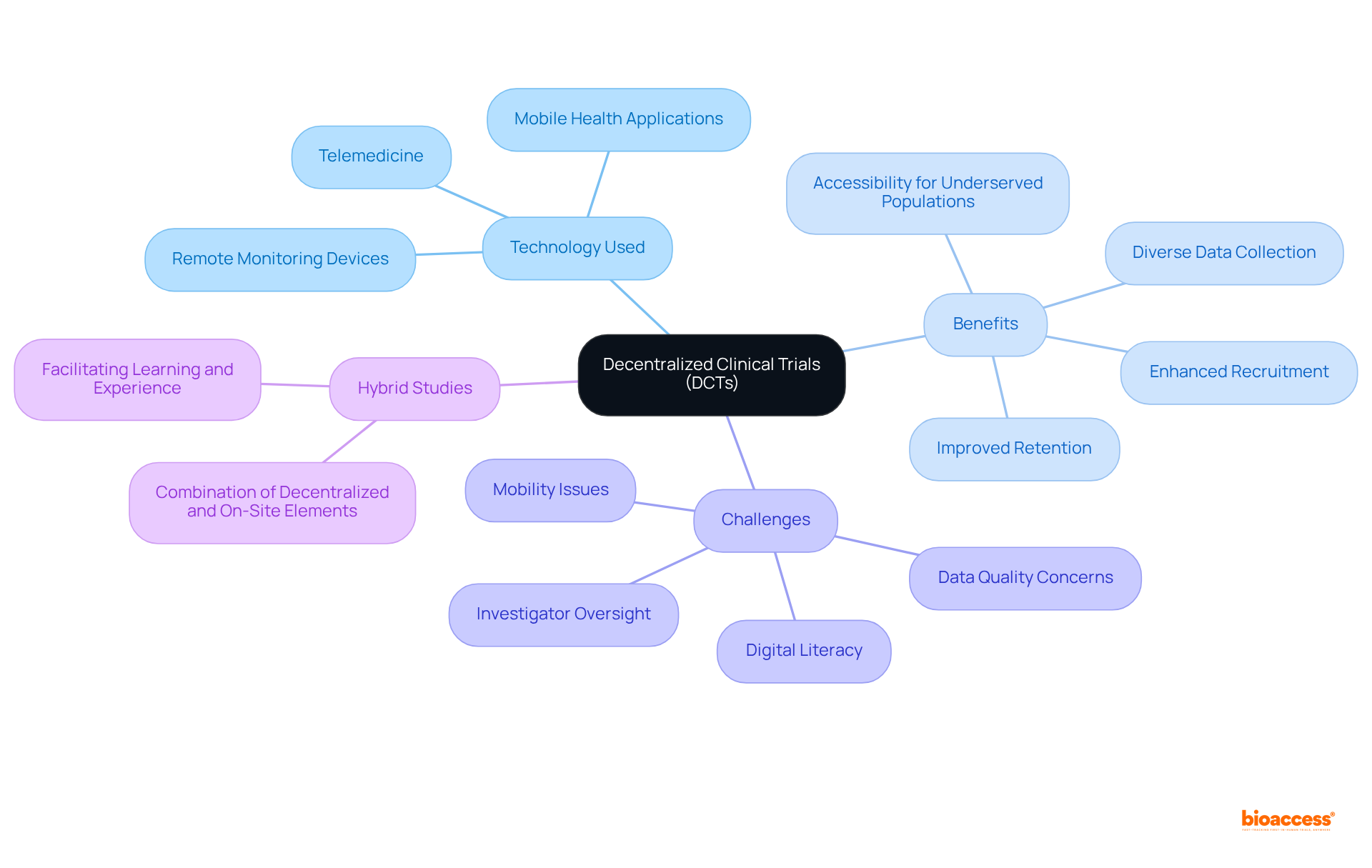
Pragmatic studies are essential for evaluating the effectiveness of interventions in real-world environments, delivering results that resonate with both individuals receiving care and healthcare providers. Unlike conventional studies, which often occur in controlled settings, pragmatic experiments assess treatment effectiveness within routine medical practice. This approach provides a comprehensive view of treatment effects by taking into account factors such as adherence, comorbidities, and variations in medical practice.
For instance, research indicates that practical evaluations can lead to a 30% increase in the relevance of research outcomes to clinical settings. The practical evidence generated from these studies is crucial, as it directly informs healthcare decisions and policies, ultimately enhancing care for individuals. Effective clinical trial designs, including adaptive methodologies, have shown their ability to bridge the gap between research and practice, ensuring that findings are both relevant and beneficial in real-world contexts.
Healthcare professionals increasingly recognize the importance of these studies, as they link medical research with the complexities of daily care, resulting in more informed and efficient healthcare strategies. As Dr. Farhana Sultana observed, 'For human papillomavirus (HPV) DNA detection, specimen collection and transportation using a dry swab without transport medium has advantages, in various situations, over liquid media.' This underscores the practical implications of pragmatic studies in improving outcomes for individuals.
To further enhance the effectiveness of pragmatic studies, research directors should consider incorporating diverse patient populations and real-world settings into their clinical trial designs.
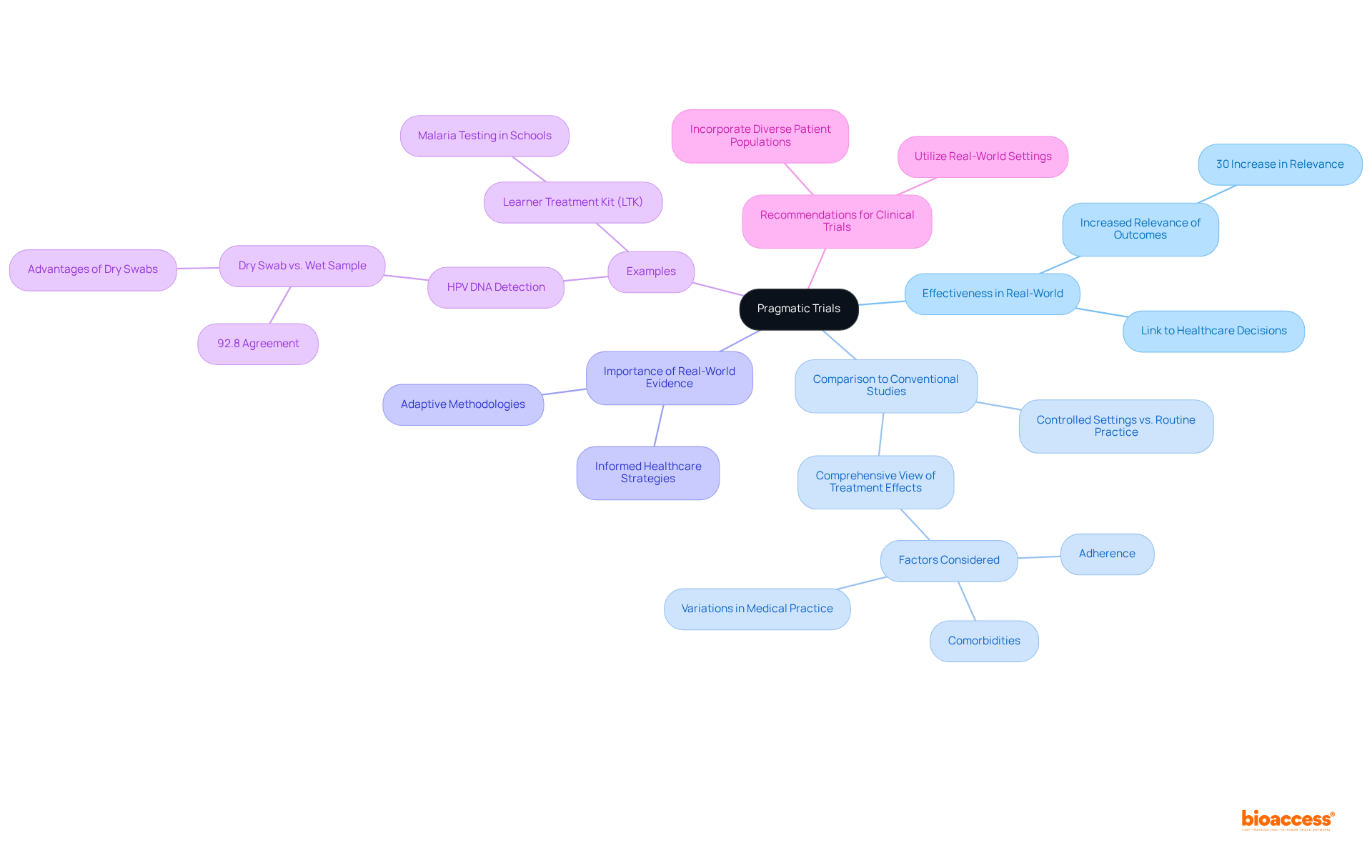
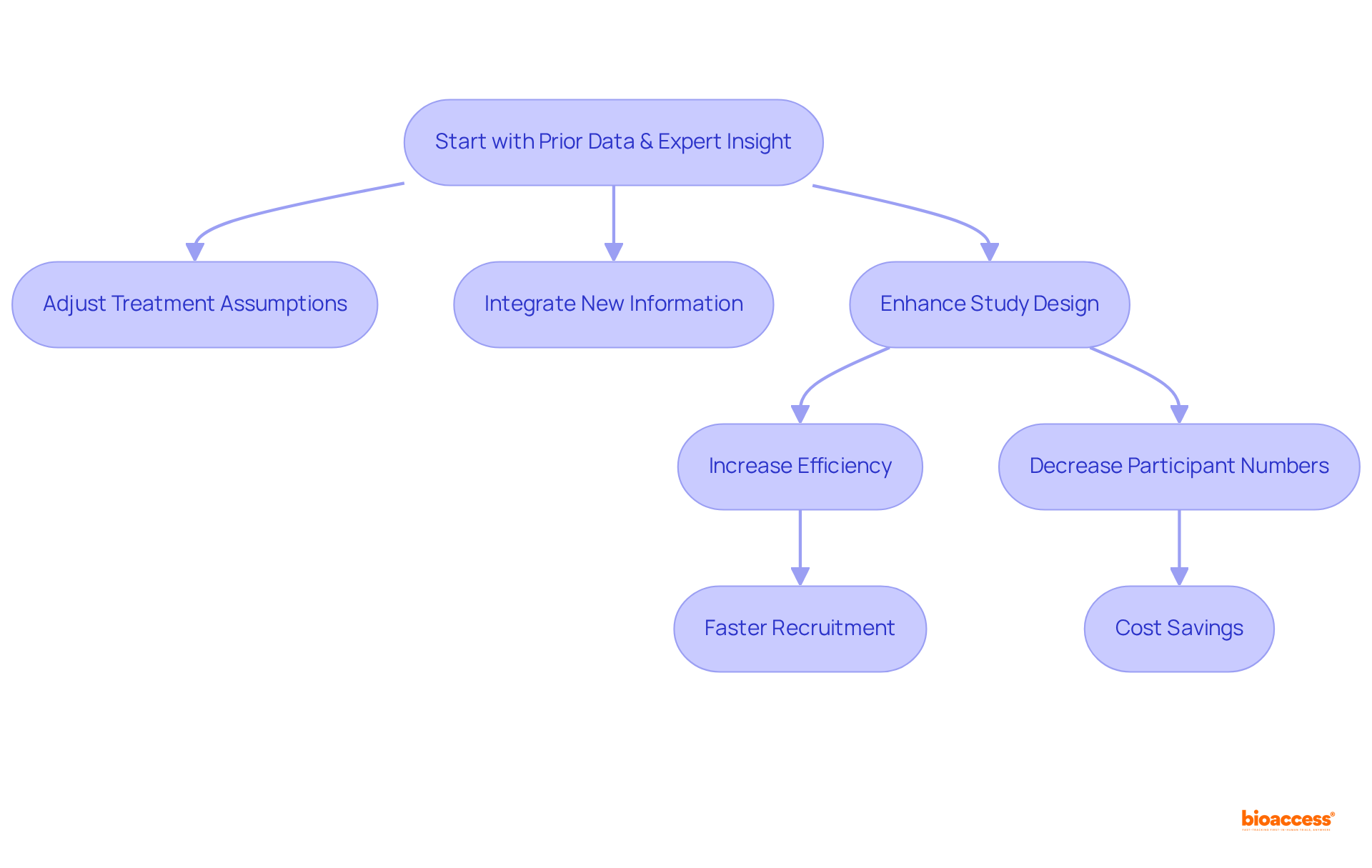
Bayesian approaches utilize previous data and expert insight to effectively guide the structure and evaluation of clinical studies. This method empowers researchers to adjust their assumptions regarding treatment effects as new information emerges, leading to more informed decision-making throughout the research process.
By integrating prior information, clinical trial designs that employ Bayesian methods can enhance the efficiency of studies, potentially decreasing the number of participants needed to achieve statistically significant results. Notably, with bioaccess®, studies can recruit treatment-naive cardiology or neurology groups 50% faster than in Western locations, resulting in substantial savings of $25K per individual with FDA-ready data—eliminating rework and delays.
This adaptability is particularly beneficial in rapidly evolving fields where new data is constantly emerging, addressing the persistent challenges of patient enrollment in early-stage clinical studies.
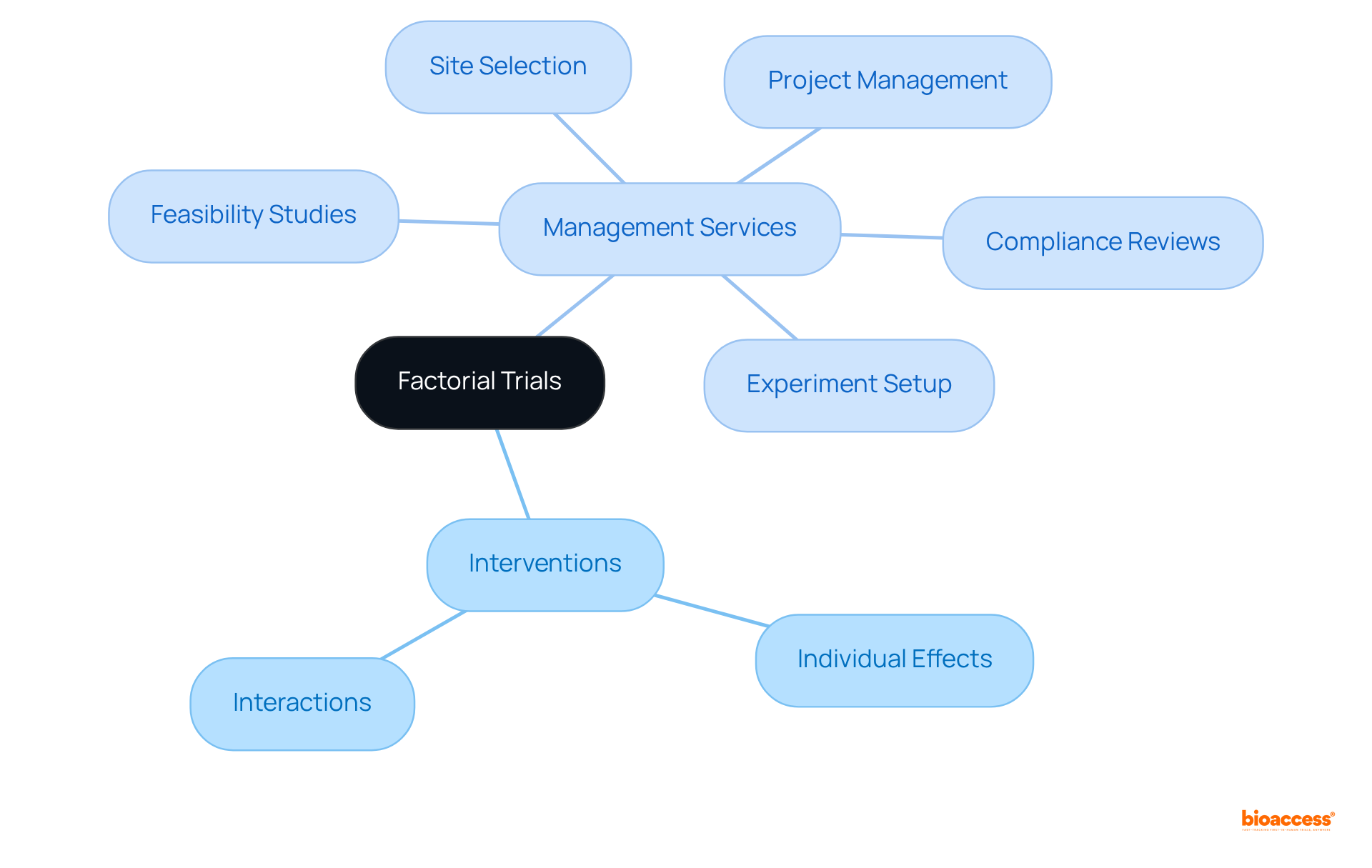
Factorial experiments are meticulously structured as clinical trial designs to evaluate the impacts of various interventions simultaneously within a single study. This design, as part of clinical trial designs, empowers researchers to assess not only the individual effects of each intervention but also the potential interactions among them. By efficiently utilizing resources and participant time, factorial studies, which are a type of clinical trial designs, provide comprehensive insights into treatment efficacy and safety.
To ensure the success of these experiments, robust management services for studies are indispensable. Services such as:
are crucial in facilitating factorial studies. This approach proves particularly beneficial in fields like oncology and chronic disease management, where multiple treatment options may exist, and effective management can significantly enhance healthcare outcomes and stimulate economic growth in local communities.
Smooth phase studies integrate various stages of medical research into a singular investigation, thereby facilitating a more effective assessment of new treatments. By merging phase II and phase III studies, researchers can accelerate the development process while upholding rigorous safety and efficacy evaluations. This design minimizes the time between phases, ultimately decreasing the overall length of development. Smooth evaluations prove especially advantageous in rapid therapeutic fields such as oncology and infectious diseases, where prompt access to new treatments is essential.
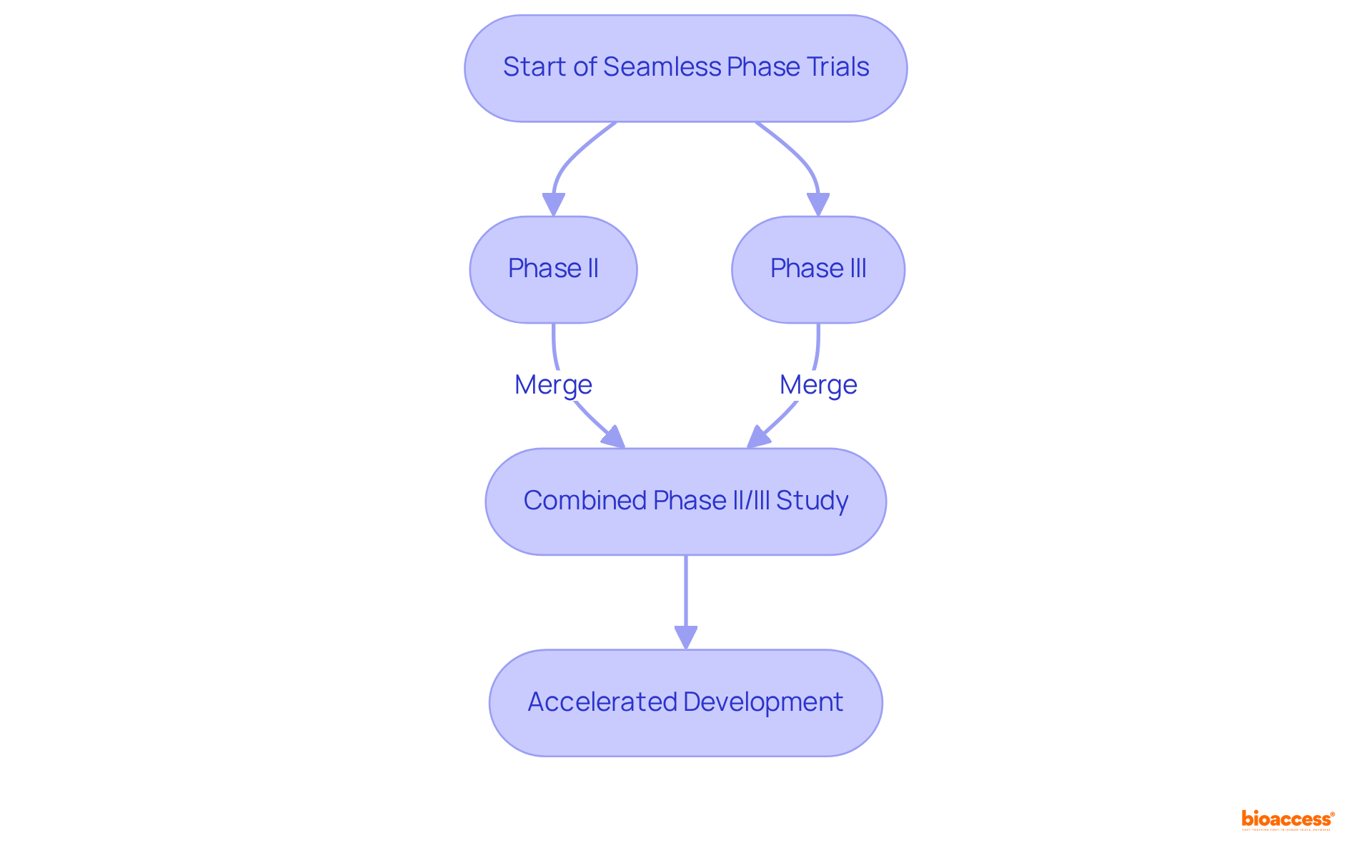
Virtual experiments leverage digital instruments and technologies to conduct research remotely, significantly enhancing flexibility and accessibility for participants. By employing telehealth, mobile applications, and remote monitoring devices, these studies not only boost patient engagement but also streamline data collection processes. This innovative design of clinical trial designs alleviates the burden on participants while broadening the scope of research, allowing diverse populations to participate in studies. As clinical research continues to evolve, virtual trials are set to play a pivotal role in shaping the future of medical research.
Innovative clinical trial designs are reshaping the landscape of medical research, presenting unparalleled opportunities to boost efficiency and enhance patient engagement. By embracing adaptive, decentralized, and seamless methodologies, organizations like bioaccess® are not only accelerating the pace of clinical trials but also enriching the participant experience. This evolution ultimately leads to expedited approvals and broader access to transformative therapies.
The article delves into various innovative designs, such as:
Each design fulfills a distinct role, from evaluating multiple therapies concurrently to harnessing technology for remote participation. These modern methodologies effectively tackle the challenges posed by traditional clinical trials, including participant recruitment and retention, while simultaneously reducing costs and timelines associated with research processes. The focus on real-world evidence and patient-centered approaches further emphasizes the critical nature of these advancements in clinical research.
As the realm of clinical trials continues to progress, the adoption of innovative designs will be essential for stakeholders striving to remain at the forefront. By recognizing the advantages of these methodologies and proactively seeking their implementation, researchers and organizations can make significant strides in advancing medical science. The future of clinical research hinges on the capacity to adapt and innovate, ensuring that effective therapies are delivered to patients more swiftly and efficiently than ever before.
What is bioaccess® and how does it impact clinical trials?
bioaccess® is a leader in clinical research that employs innovative trial designs to simplify research processes, reducing the time and resources needed for studies. Their adaptive, decentralized, and seamless methodologies enhance trial efficiency, ensuring rapid patient recruitment and efficient data collection, which facilitates expedited approvals and faster market access for medical technologies and therapies.
What challenges do decentralized studies address for participants?
Decentralized studies help tackle travel challenges for participants, as 44% of potential participants find traveling to study clinics difficult. This approach enhances overall engagement and improves participant experience.
What is the projected growth for electronic data capture systems in clinical research?
The integration of advanced technologies, such as electronic data capture systems, is projected to grow at a compound annual growth rate of 14.6% from 2023 to 2030, reflecting a shift towards more efficient research practices in clinical trial designs.
How do basket clinical trials work and what are their benefits?
Basket clinical trials evaluate the effectiveness of various treatments targeting a single condition within a cohesive framework. This design is particularly beneficial in oncology, allowing researchers to group individuals with different tumor types, which significantly reduces time and costs associated with conventional trials while enhancing the understanding of treatment effects across diverse populations.
What is the Basket of Baskets (BoB) study?
The Basket of Baskets (BoB) study has successfully treated over 170 individuals, demonstrating the effectiveness of basket clinical trial designs in providing personalized therapy options.
How can basket clinical trial designs reduce research expenses?
The implementation of basket studies can lead to a 20-30% reduction in total research expenses, making them an attractive option for Medtech firms navigating complex research processes.
What initiative was announced between bioaccess™ and Caribbean Health Group?
On March 29, 2019, bioaccess™ and Caribbean Health Group announced a partnership to establish Barranquilla, Colombia, as a key location for medical studies in Latin America. This initiative aims to enhance ambulatory services for research, including feasibility studies, site selection, compliance reviews, and project management, while fostering local economic growth.
What are adaptive trial designs and their advantages?
Adaptive trial designs allow investigators to make real-time modifications to protocols based on interim data, including adjusting dosages, altering participant groups, and discontinuing ineffective treatments. This flexibility enhances study ethics and efficiency, enabling faster enrollment and significant cost savings per patient, ultimately accelerating the path to market for new treatments.