


The article titled "9 Key Insights from the MAGI Conference 2025 for Clinical Research" highlights crucial takeaways that can significantly impact clinical research practices. This conference serves as a pivotal platform for discussing the evolving landscape of Medtech and the essential role of collaboration, diversity, and innovative strategies in enhancing research efficiency and effectiveness.
In the Medtech sector, the insights shared at the MAGI Conference are particularly relevant. They address key challenges faced by clinical researchers today, emphasizing the need for a collaborative approach that leverages diverse perspectives. By fostering an environment of innovation, stakeholders can navigate complexities and drive advancements in clinical research.
Ultimately, the importance of collaboration cannot be overstated. As the Medtech field continues to evolve, embracing these insights will be vital for researchers aiming to improve outcomes and streamline processes. Moving forward, it is essential for professionals in the field to consider how they can implement these strategies in their own practices.
The landscape of clinical research is rapidly evolving, driven by innovative strategies and collaborative efforts that address the pressing needs of the Medtech sector. As we approach the MAGI Conference 2025, key insights emerge, underscoring the importance of:
But how can organizations effectively navigate these complexities to enhance their research outcomes and ultimately drive medical advancements? This article delves into nine pivotal insights from the conference, offering a roadmap for clinical research success in a competitive and dynamic environment.
This company emerges as a leader in the research field, particularly in advancing medical technology. By strategically positioning itself in Latin America, the Balkans, and Australia, it accelerates the research process, allowing medical technology firms to expedite their market entry. The organization’s steadfast commitment to ethical practices and regulatory compliance ensures that innovations are not only fast-tracked but also adhere to the highest standards of safety and efficacy. This dual focus on speed and quality establishes it as an essential ally for Medtech innovators navigating the complex landscape of research studies.
Moreover, the rapidly evolving regulatory environment in these regions enhances success rates in studies, positioning the company as a vital partner in achieving medical milestones effectively. As Medtech firms face increasing challenges in clinical research, collaboration with a trusted partner like this organization becomes crucial. With its expertise and strategic insights, the company is well-equipped to address the pressing needs of the industry, fostering innovation and ensuring compliance.
In summary, the importance of collaboration in the Medtech sector cannot be overstated. By partnering with this company, innovators can not only streamline their research processes but also enhance their chances of success in a competitive market. The next step is clear: engage with a leader in the field to navigate the complexities of clinical research and drive medical advancements forward.
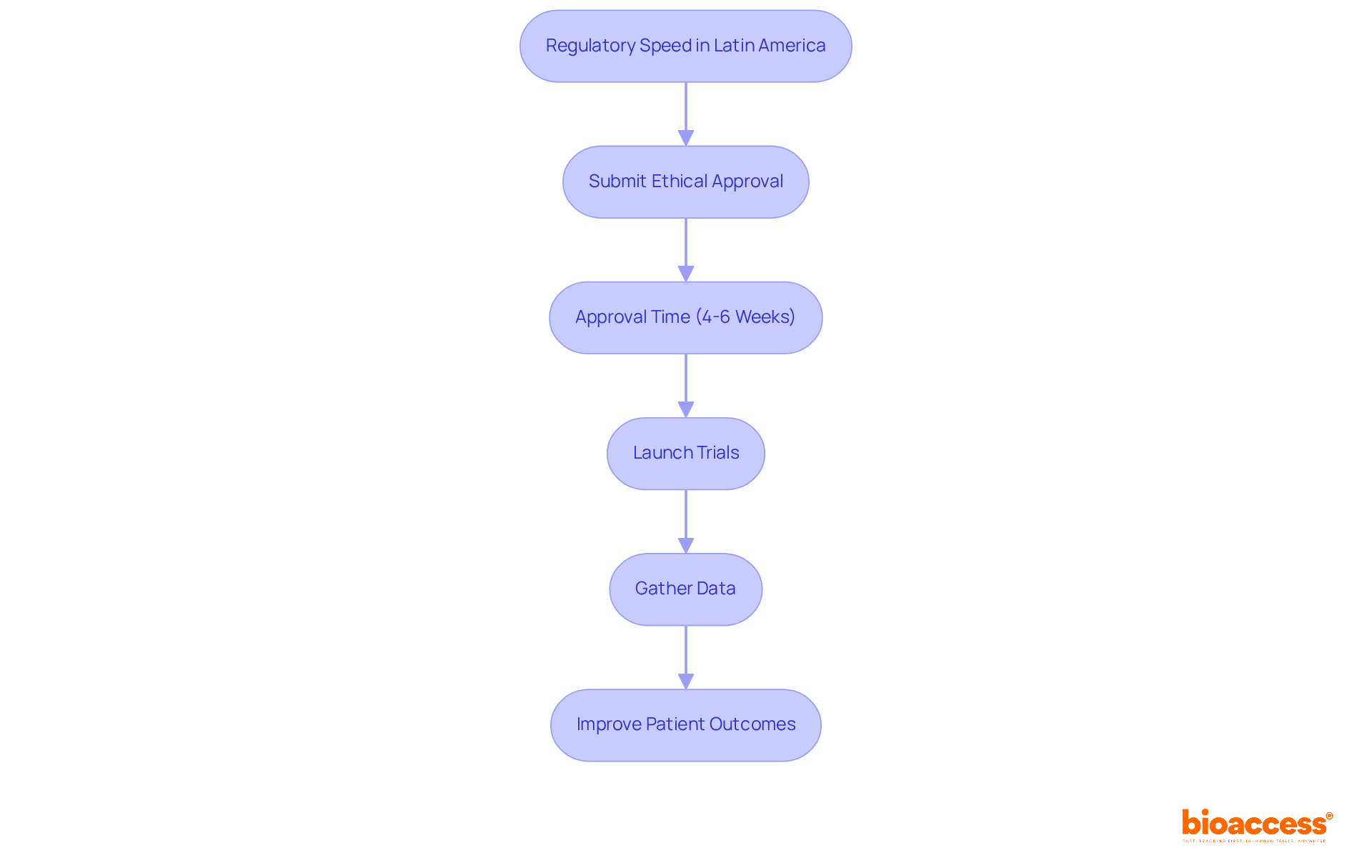
Latin America presents a distinctive regulatory environment that accelerates the clinical research process significantly. Countries in this region, particularly Colombia, have optimized their approval processes, enabling organizations to secure ethical approvals in just 4-6 weeks. This remarkable regulatory speed is a game-changer for medical technology companies, allowing them to launch trials and gather data much more quickly than in traditional markets, where the average trial duration can extend considerably. By leveraging these advantages, bioaccess® empowers clients to shorten their time-to-market, thereby enhancing their competitive edge in the global Medtech landscape.
The streamlined procedures not only facilitate quicker access to innovative treatments but also improve patient outcomes. This makes Latin America an increasingly attractive destination for research trials. As the Medtech sector continues to evolve, collaboration with experienced partners like bioaccess® becomes essential for navigating these opportunities effectively. Are you ready to explore how these advantages can transform your clinical research strategy?
The Balkans present a rich and diverse patient demographic, crucial for advancing medical research. This diversity allows bioaccess® to significantly enhance the strength of research studies, ensuring that findings are relevant across various populations. Such demographic variety not only strengthens the generalizability of results but also helps identify potential differences in treatment responses. For Medtech companies, this means more comprehensive data that can effectively support regulatory submissions and market access strategies.
As we approach the magi conference 2025, the emphasis on demographic diversity in research studies is gaining recognition, with regulatory bodies increasingly endorsing inclusive enrollment strategies. This shift is vital for addressing health disparities and ensuring that research accurately reflects the populations it aims to serve. Ultimately, this approach leads to improved healthcare outcomes, underscoring the importance of collaboration in the Medtech landscape.
How can your organization leverage this demographic diversity to enhance your research efforts?
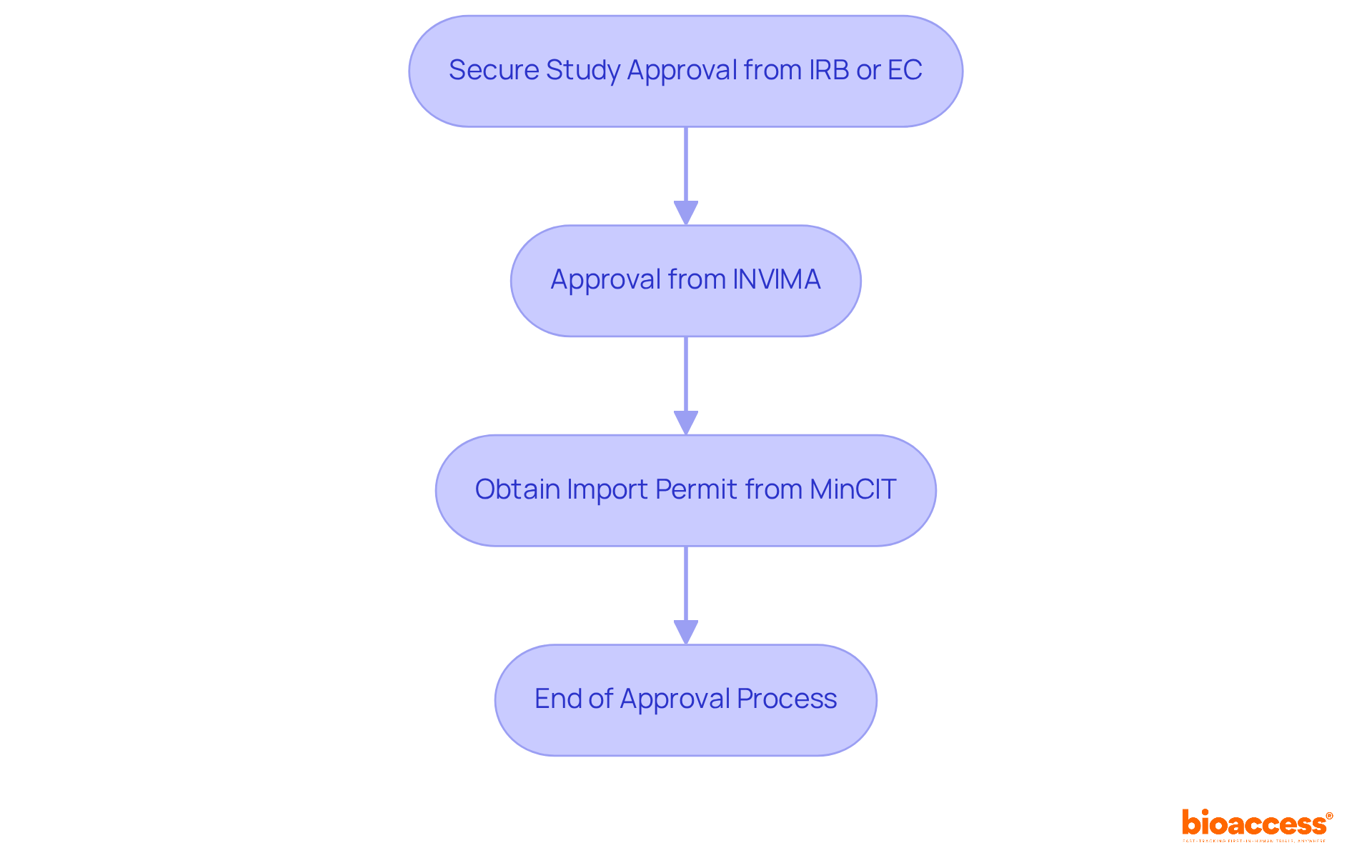
Obtaining ethical approvals is often one of the most time-consuming phases of clinical trials. However, bioaccess® has implemented streamlined processes that significantly reduce the time required for these approvals. By fostering strong relationships with ethics committees and leveraging local expertise, bioaccess® ensures that ethical considerations are addressed swiftly and effectively. This proactive approach not only accelerates testing timelines but also enhances the overall integrity of the research process, instilling confidence in stakeholders and participants alike.
In Colombia, the process begins with securing study approval from the site's institutional review board (IRB) or ethics committee (EC), followed by approval from Colombia's regulatory agency (INVIMA) and obtaining an import permit from the Ministry of Industry and Commerce (MinCIT). Notably, studies show that the introduction of selected governance and ethics processes can lead to shorter approval times. For instance, median ethics approval times decreased from 46 days pre-pandemic to 42 days post-pandemic in Australia. Furthermore, the overall median time to site activation was 234 days, with ethics approval taking a median of 48 days and governance approval a median of 34 days.
Organizations that prioritize mutual acceptance of ethics approvals have demonstrated shorter overall activation times, underscoring the value of collaboration in expediting clinical research. This collaborative spirit not only streamlines processes but also enhances the credibility of the research, making it imperative for stakeholders to consider how they can engage in such partnerships.
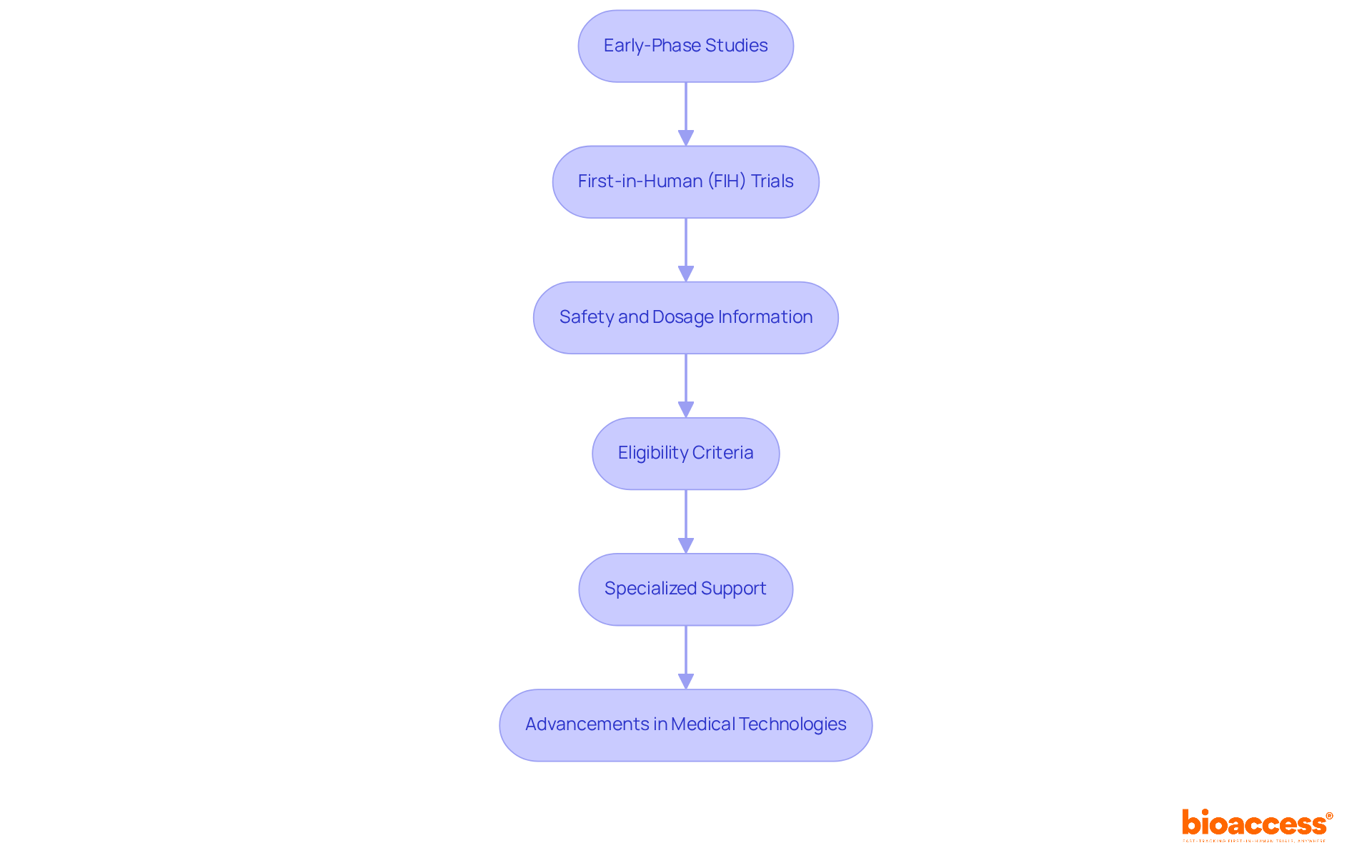
Early-phase studies, particularly first-in-human (FIH) experiments, play a pivotal role in advancing Medtech innovations. These studies are essential for providing critical safety and dosage information, laying the groundwork for subsequent research stages. Over the years, the complexity of FIH trials has escalated, with eligibility criteria becoming more stringent, which underscores the need for specialized support. Bioaccess® excels in facilitating these early-phase studies, ensuring they are executed with both efficiency and ethical rigor. By leveraging extensive local expertise and resources, bioaccess® adeptly navigates the intricacies of early-phase research, thereby accelerating the development of groundbreaking medical technologies.
Successful instances of FIH studies underscore their importance; for instance, research in oncology and infectious diseases has demonstrated significant advancements in treatment alternatives. Expert insights reveal that robust safety data from these trials is vital for informed decision-making in later phases, ultimately enhancing patient outcomes and fostering innovation in the medical technology sector. As we look to the future, collaboration among stakeholders will be crucial in overcoming challenges and driving progress in clinical research.
Latin America is emerging as a dynamic healthcare market, presenting substantial opportunities for innovations in medical technology. This service plays a crucial role in helping clients navigate this intricate landscape through comprehensive market access services. By leveraging a deep understanding of local regulations and reimbursement policies, the company empowers medical technology firms to strategically position their products and capitalize on emerging opportunities. This strategy not only enhances the likelihood of successful product launches but also fosters sustainable growth in the region.
For example, companies that prioritize local relationships and expertise are more likely to succeed, as seen in the growing investments in telemedicine and digital health solutions. Moreover, effective communication with regulatory bodies like ANVISA is essential for compliance and market entry, enabling companies to adapt to evolving requirements and maintain a competitive edge. With the Medtech sector in Latin America projected to reach approximately USD 30 billion by 2025, the strategic insights provided are invaluable for navigating this transformative market.
Clinical research encounters significant challenges, especially in patient recruitment and regulatory compliance. Addressing these hurdles, bioaccess® effectively leverages local networks and innovative recruitment strategies, significantly enhancing patient engagement and retention. In collaboration with Caribbean Health Group, bioaccess® aims to position Barranquilla as a premier hub for medical research in Latin America, a vision supported by Colombia's Minister of Health. This initiative is designed to attract more research projects to the region, thereby enriching the overall environment for studies.
Statistics reveal that:
To combat these challenges, bioaccess® employs:
Furthermore, through its partnership with GlobalCare Clinical Trials, bioaccess® has achieved over a 50% reduction in recruitment time and an impressive 95% retention rate.
The organization’s deep understanding of regulatory requirements, combined with advancements in artificial intelligence and machine learning to anticipate patient behavior, empowers it to proactively tackle compliance challenges. This approach not only reduces delays but also enhances the overall effectiveness of research studies. By focusing on both recruitment and compliance, bioaccess® simplifies processes and fosters a more patient-centered approach, ultimately leading to improved outcomes in research.
Cooperation with Contract Research Organizations (CROs) is crucial for the success of research studies. bioaccess® exemplifies how effective collaborations can lead to improved outcomes, particularly in first-in-human medical device studies in Colombia. By providing comprehensive CRO research services—including study design, feasibility evaluations, regulatory submissions, project management, study monitoring, and reporting—bioaccess® accelerates the research process for medical technology and biopharma startups across Latin America, Eastern Europe, and Australia. This collaborative approach enhances operational efficiency and fosters innovation, ultimately resulting in more successful medical studies and quicker market entry for Medtech products.
Notably, nearly 75% of research studies are conducted by CROs, underscoring their ability to enhance study efficiency and increase productivity. As Michael Jordan aptly stated, "Talent wins games, but teamwork and intelligence win championships," which captures the essence of successful CRO partnerships. Furthermore, effective communication within these collaborations significantly impacts study outcomes, as 51% of drug developers acknowledge the increasing complexity of clinical studies as a primary challenge. By prioritizing collaboration, the company not only navigates these challenges but also positions its clients for success in a competitive market.
Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, reinforces this point by sharing his positive experience with the product during its initial human trial in Colombia, highlighting the critical role of such collaborations.
In the competitive realm of clinical research, achieving cost-effectiveness is not just beneficial; it’s essential for success. This company provides innovative solutions that empower medical technology firms to enhance value while effectively reducing costs. By leveraging local resources, optimizing study designs, and implementing robust project management practices, bioaccess® enables clients to meet their research objectives without sacrificing quality. This commitment to cost-efficiency not only bolsters the viability of medical studies but also fosters the long-term sustainability of healthcare innovations.
Moreover, efficient budget management is crucial. Regular evaluations and a thorough understanding of all cost categories—including personnel, patient care, and regulatory expenses—are vital for identifying inefficiencies and ensuring that every budget item contributes value to the study. By adopting a patient-focused strategy and utilizing cutting-edge technologies, bioaccess® illustrates how Medtech firms can navigate the complexities of research studies while maximizing their return on investment.
In this landscape, collaboration is key. As the Medtech sector continues to evolve, companies must consider how they can partner with experts like bioaccess® to tackle their unique challenges in clinical research.
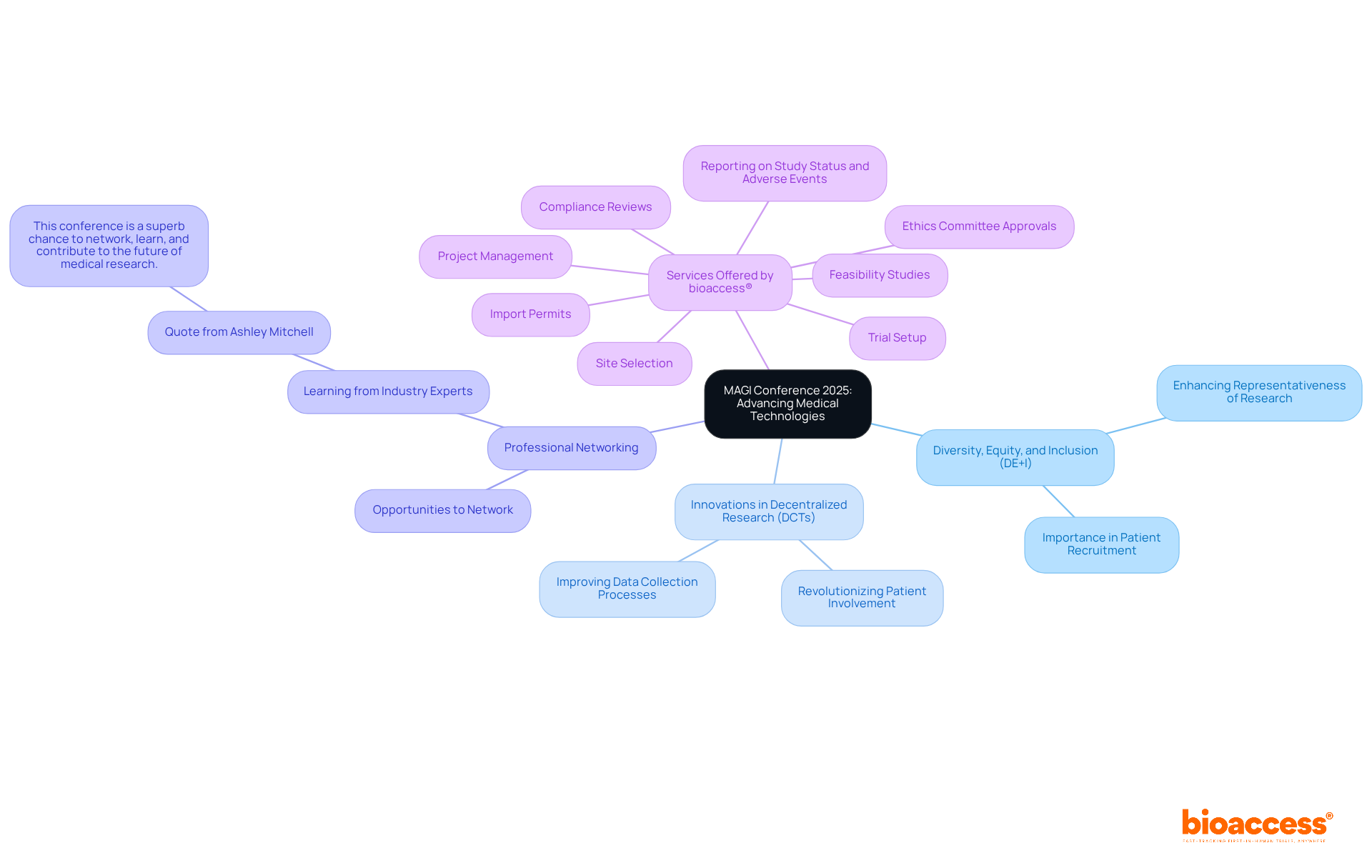
The magi conference 2025 serves as a pivotal platform for advancing medical technologies and research practices. By uniting industry leaders, researchers, and regulatory experts, the conference ignites discussions on best practices, innovative strategies, and emerging trends. A key theme is the increasing emphasis on Diversity, Equity, and Inclusion (DE+I) in patient recruitment, which is essential for enhancing the representativeness of research studies. Innovations showcased at the conference, particularly advancements in decentralized research studies (DCTs), are set to revolutionize patient involvement and data collection processes.
Insights from experts highlight the necessity of aligning metrics with team performance to cultivate a positive work environment. As Ashley Mitchell noted, "This conference is a superb chance to network, learn, and contribute to the future of medical research." Furthermore, bioaccess® stands out as a US-based CRO committed to bridging medical technology innovation and clinical research in Latin America. They offer a comprehensive suite of clinical trial management services, including:
Participation in such conferences not only broadens knowledge but also fortifies professional networks, ultimately benefiting the wider Medtech community. For more information and to register, visit the magi conference 2025 website.
The insights gathered from the MAGI Conference 2025 underscore the significant potential of strategic partnerships and innovative practices in clinical research, especially within the Medtech sector. By prioritizing collaboration, leveraging regulatory advantages, and embracing diverse patient demographics, organizations can markedly enhance their research capabilities and accelerate the development of medical technologies.
Key discussions highlighted the value of harnessing Latin America's regulatory speed alongside the rich patient diversity found in the Balkans. Together, these factors contribute to more efficient and representative clinical trials. Additionally, streamlining ethical approvals and fostering cooperation with Contract Research Organizations (CROs) emerged as essential strategies for overcoming prevalent challenges in clinical research, such as recruitment and compliance issues. The focus on cost-effective solutions further emphasizes the necessity for Medtech firms to maximize value while navigating complex research landscapes.
Ultimately, the MAGI Conference 2025 serves as a crucial reminder of the collaborative spirit essential for advancing medical technologies. Engaging with industry leaders and adopting innovative strategies not only propels progress but also ensures that the research conducted remains relevant, ethical, and impactful. As the Medtech sector continues to evolve, stakeholders are urged to explore these insights and consider how they can implement similar strategies to enhance their own clinical research efforts, paving the way for future breakthroughs in healthcare.
What is bioaccess® and its role in clinical research for Medtech innovations?
bioaccess® is a leader in the research field that accelerates clinical research for medical technology innovations. It strategically operates in Latin America, the Balkans, and Australia to expedite market entry for Medtech firms while ensuring adherence to ethical practices and regulatory compliance.
How does bioaccess® ensure the quality of its research processes?
The organization maintains a steadfast commitment to ethical practices and regulatory compliance, ensuring that innovations are fast-tracked while adhering to the highest standards of safety and efficacy.
Why is collaboration with bioaccess® important for Medtech firms?
Collaboration with bioaccess® helps Medtech firms navigate the complex landscape of clinical research, addressing pressing industry needs and enhancing their chances of success in a competitive market.
What advantages does Latin America offer for clinical trials?
Latin America, particularly Colombia, has optimized its regulatory environment, allowing organizations to secure ethical approvals in just 4-6 weeks. This regulatory speed significantly accelerates the clinical research process compared to traditional markets.
How does regulatory speed in Latin America benefit Medtech companies?
The streamlined approval processes enable Medtech companies to launch trials and gather data more quickly, shortening their time-to-market and enhancing their competitive edge in the global Medtech landscape.
What is the significance of diverse patient pools in the Balkans for clinical research?
The Balkans offer a rich and diverse patient demographic, which enhances the strength and generalizability of research studies. This diversity helps identify potential differences in treatment responses and supports regulatory submissions and market access strategies.
How is the emphasis on demographic diversity in research studies changing?
Regulatory bodies are increasingly endorsing inclusive enrollment strategies, recognizing the importance of demographic diversity in addressing health disparities and ensuring research reflects the populations it aims to serve.
What is the impact of demographic diversity on healthcare outcomes?
Emphasizing demographic diversity in research leads to improved healthcare outcomes by ensuring that findings are relevant across various populations and addressing potential treatment response differences.