


The article titled "9 Strategies for Successful Formulation Drug Development" primarily aims to delineate effective strategies that pharmaceutical companies can adopt to optimize the drug formulation process. It articulates nine pivotal strategies, such as:
These strategies collectively enhance the efficiency of drug development and significantly increase the likelihood of successful market entry.
The landscape of drug development is evolving rapidly, with innovative strategies emerging to streamline the formulation process and enhance market readiness. As companies strive to navigate complex regulatory environments and meet increasing patient demands, understanding effective drug formulation strategies becomes paramount.
What are the key approaches that can not only accelerate drug development but also ensure compliance and patient satisfaction in an ever-competitive market? Exploring these strategies reveals critical insights that empower pharmaceutical innovators to thrive in the future of healthcare.
bioaccess® excels in delivering rapid clinical research services that significantly reduce the drug development timeline. By leveraging Latin America's regulatory speed, the Balkans' diverse patient populations, and Australia's efficient ethical approval processes, bioaccess® achieves ethical approvals in just 4-6 weeks. This capability enables patient enrollment rates that are 50% faster than traditional markets, a critical advantage for Medtech, Biopharma, and Radiopharma innovators aiming to expedite their products' market entry.
Recent advancements in clinical research underscore the importance of this agility. For instance, the FDA's shift towards accepting clinical trial data from Mexican sites, contingent on adherence to Good Clinical Practice, reflects a growing recognition of the region's potential. bioaccess® is strategically positioned to facilitate compliance with these new standards, ensuring that clients can navigate the evolving regulatory landscape effectively. Furthermore, studies conforming to SPIRIT guidelines have demonstrated superior quality metrics, enhancing the overall integrity of research outcomes, which aligns with bioaccess®'s commitment to high-quality clinical research.
The benefits of rapid clinical research extend beyond speed; they encompass cost-effectiveness and improved patient engagement. With Mexico's favorable exchange rates and lower operational costs, U.S.-based companies can significantly reduce their clinical research budgets. This financial advantage, combined with the increasing incidence of lifestyle-related diseases in Mexico, creates a robust environment for patient recruitment. Notably, 30% of clinical studies experience delays due to insufficient resource planning, highlighting the critical role of bioaccess®'s rapid services in mitigating such risks.
Expert opinions emphasize that the integration of decentralized trial structures, although initially challenging, often yields substantial benefits, including enhanced participant diversity and faster enrollment. bioaccess® is actively adapting to these trends, ensuring that their clients can leverage the advantages of decentralized trials. Successful examples of expedited ethical approvals, such as bioaccess®'s ability to navigate complex regulatory landscapes, further illustrate the effectiveness of their approach in facilitating timely treatment development. By concentrating on these essential components, bioaccess® empowers innovators to introduce their revolutionary therapies to consumers more effectively.
Navigating the regulatory environment is crucial for successful medication development. Companies must thoroughly understand the specific requirements established by regulatory bodies such as the FDA and EMA. This encompasses a comprehensive grasp of:
Early collaboration with regulatory advisors can identify potential obstacles and enhance compliance initiatives, ultimately accelerating time to market.
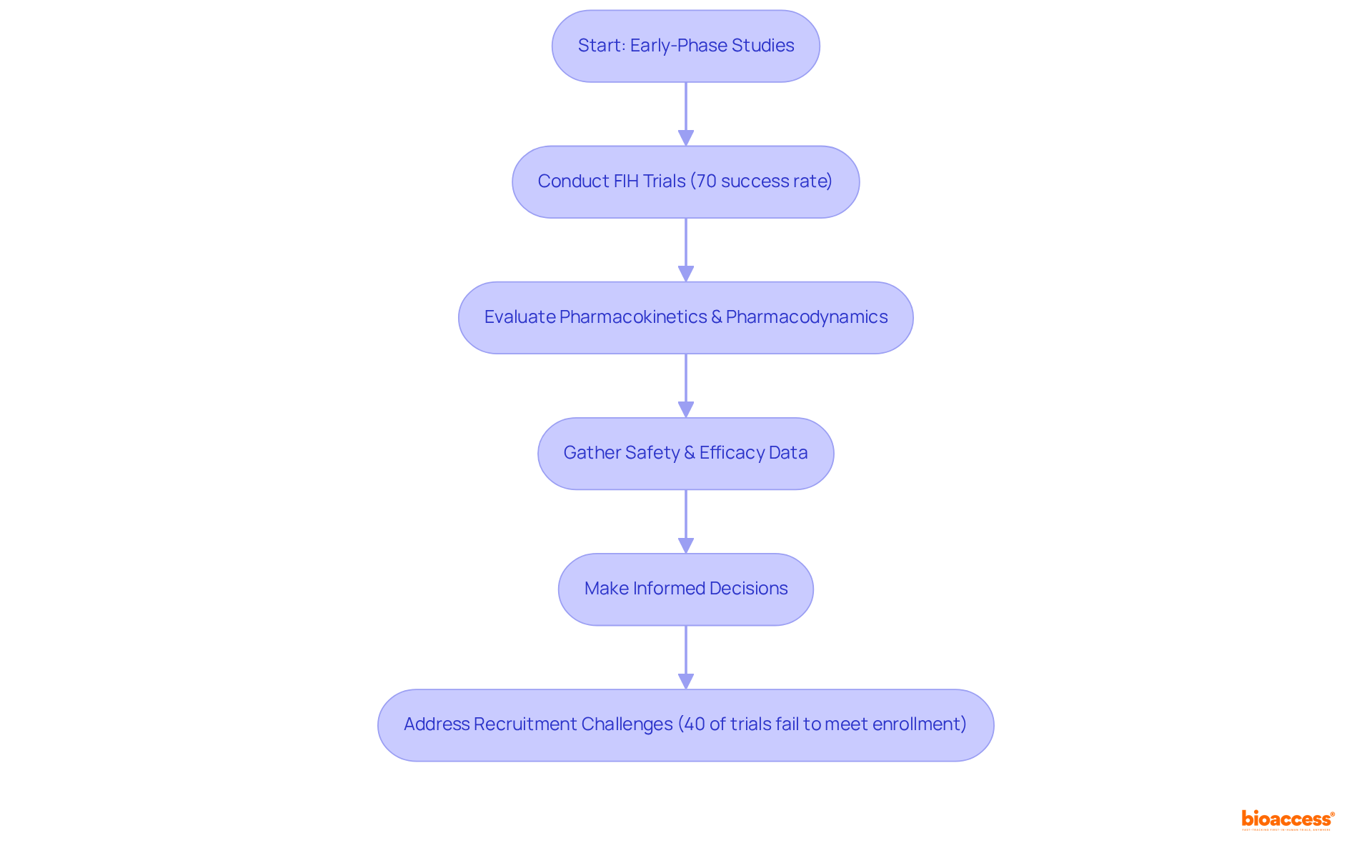
Carrying out early-phase research, such as First-in-Human (FIH) and Early-Feasibility Studies (EFS), is essential for validating drug development strategies. These studies enable researchers to evaluate the pharmacokinetics and pharmacodynamics of new formulation drugs within a controlled environment. By gathering safety and efficacy data early in the development process, companies can make informed decisions regarding further development and potential market entry. Notably, approximately 70% of drugs in FIH trials successfully progress to later phases, highlighting the critical role these studies play in shaping future drug development pathways.
For example, Avantec Vascular's first-in-human trial in Latin America, supported by bioaccess®, resulted in a 50% increase in patient enrollment for subsequent phases, thanks to robust safety data. This underscores how effective FIH investigations can streamline the development process and expedite market access.
Furthermore, the incorporation of real-world data and adaptive methodologies in EFS has demonstrated promising results, allowing for real-time adjustments based on interim findings. The growing adoption of Bayesian statistics in clinical evaluations reflects innovative approaches that enhance the efficiency of EFS. As Vivienne van der Walle aptly noted, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care." These early-stage studies not only validate formulation drug compositions but also enhance the overall effectiveness of clinical trials, ultimately leading to improved patient care and treatment options.
However, significant challenges in participant recruitment persist, with nearly 40% of first-in-human investigations failing to meet enrollment targets. Addressing these challenges is vital for maximizing the potential of early-phase studies.
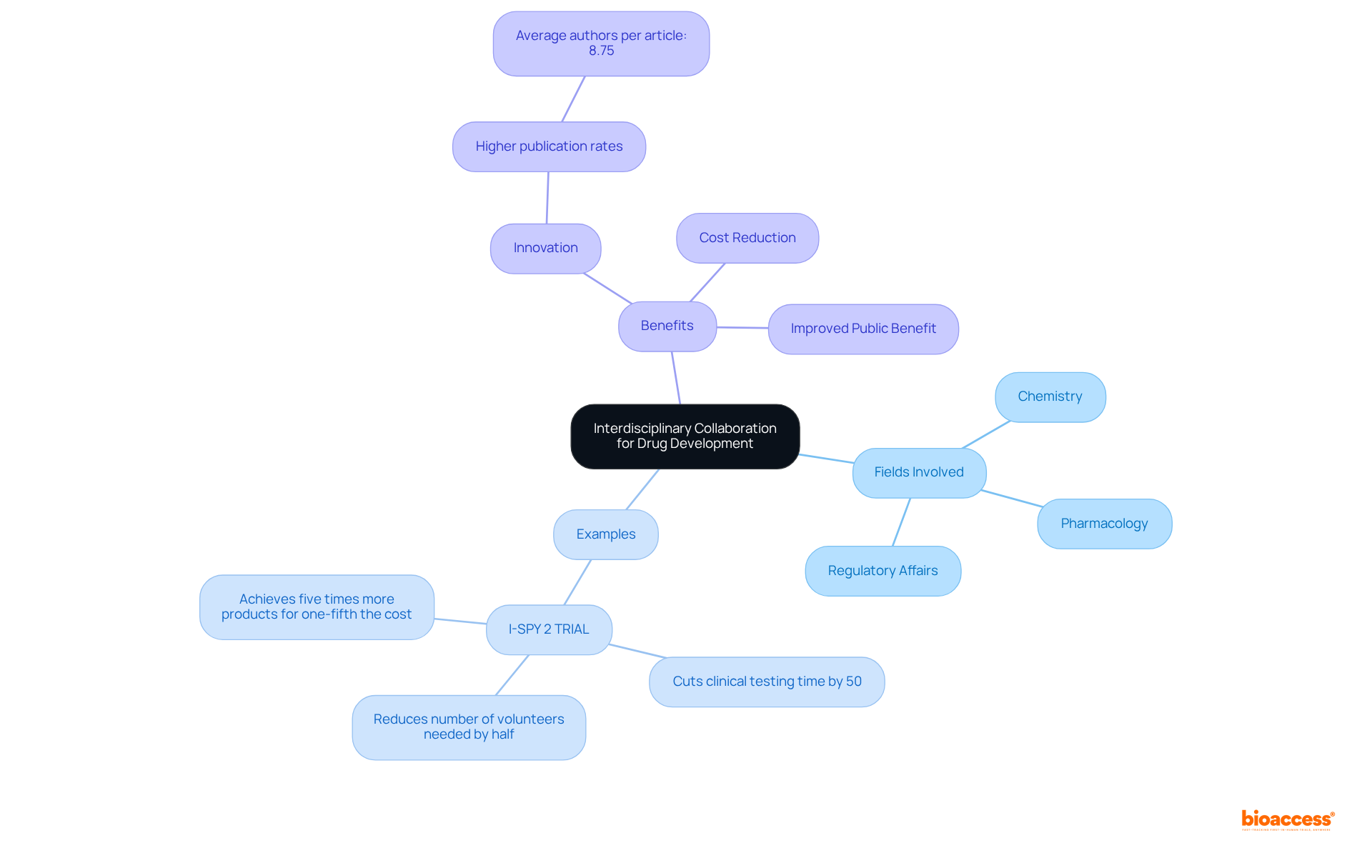
Encouraging interdisciplinary cooperation is essential for effective drug development. By uniting specialists from diverse fields—such as chemistry, pharmacology, and regulatory affairs—companies can harness a wealth of perspectives that enhance strategic development. Collaborative efforts have consistently demonstrated their ability to yield innovative solutions for complex formulation drug challenges, significantly boosting the likelihood of successful product development.
For instance, the I-SPY 2 TRIAL aims to cut clinical testing durations for new medications by fifty percent, exemplifying how cooperative platforms can foster the exchange of insights among various research organizations. Moreover, studies reveal that diverse expertise within teams correlates with elevated publication rates, averaging 8.75 authors per article, which underscores the collaborative essence of successful research.
Engaging in cross-disciplinary collaboration not only accelerates the formulation drug development process but also cultivates an environment where innovative ideas can flourish, ultimately benefiting the entire pharmaceutical sector. As noted by experts, 'Collaborations will reduce costs and our best efforts will be put into the collaboration, which will result in improved benefit to the public.'
This approach aligns seamlessly with bioaccess®'s commitment to facilitating such collaborations, leveraging its extensive experience and market access services across Latin America, Australia, and the Balkans.
Adopting patient-focused strategies in medication development is essential for creating products that resonate with end-users. Engaging patients through surveys and focus groups provides invaluable insights into their preferences and experiences. Notably, research indicates that incorporating patient feedback can lead to a 30% increase in retention rates during trials. By integrating this feedback into the development process, companies can produce products that are not only effective but also user-friendly, significantly enhancing patient adherence and satisfaction.
Furthermore, treatments developed with patient-focused methods boast an impressive 87% success rate in achieving commercialization, compared to just 68% for those lacking such approaches. This highlights the critical role of patient involvement in shaping formulation drug processes that genuinely meet their needs.
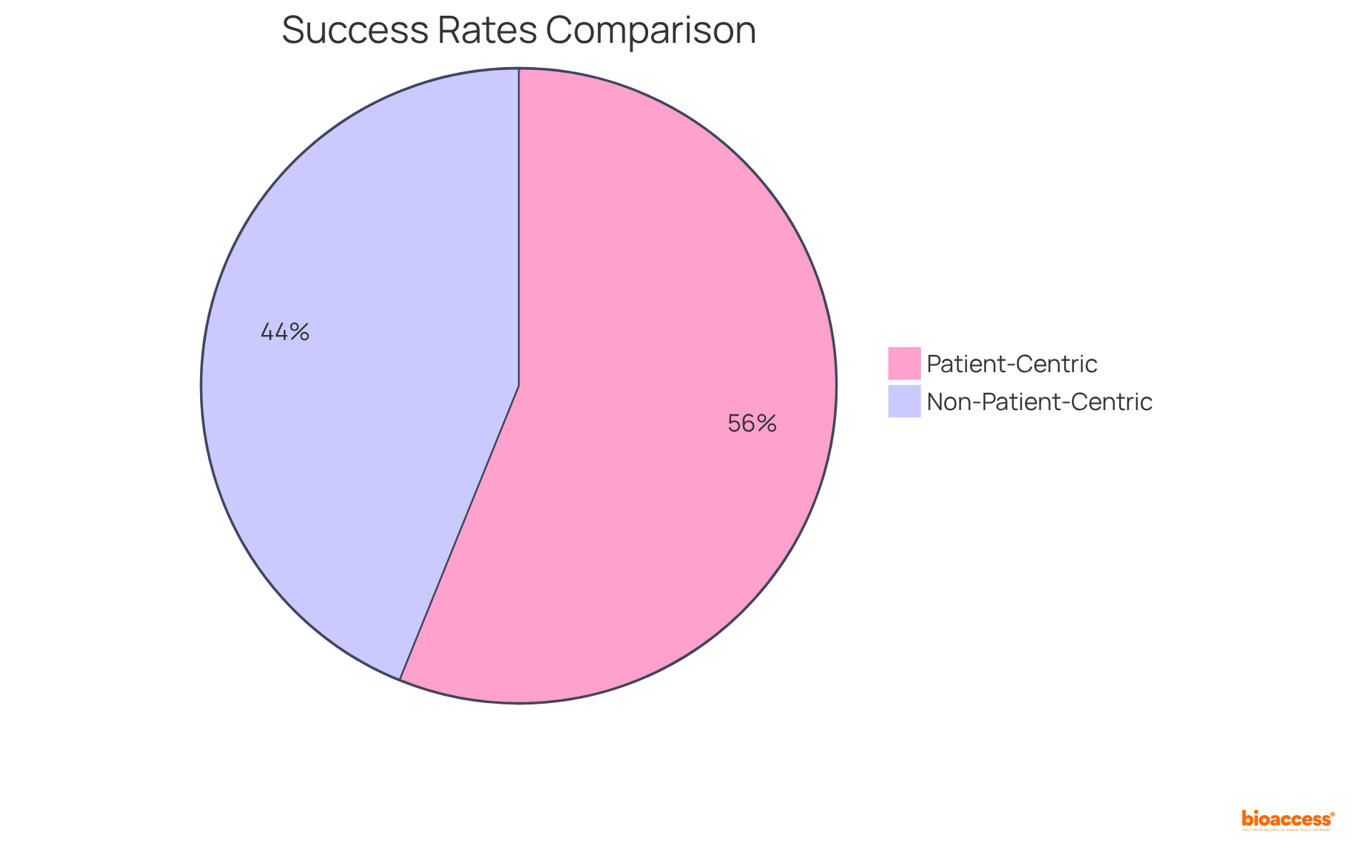
Establishing strong access strategies is essential for the successful introduction of new pharmaceuticals. Companies must conduct comprehensive industry research to grasp payer dynamics, pricing strategies, and reimbursement pathways. Involving stakeholders early in the development process can reveal potential obstacles to access and influence strategies that align with consumer needs. Notably, 57% of drug launch failures arise from restricted access, while 47% are linked to insufficient comprehension of demand and customer requirements. This underscores the importance of thorough planning.
Furthermore, 75% of pharmaceutical companies have integrated Health Economics and Outcomes Research (HEOR) into their strategic decision-making, with investment in HEOR by biotech firms increasing by 65% over the last decade. Effective instances demonstrate that aligning product value with payer expectations can greatly improve entry success, ultimately enabling smoother product launches and maximizing the potential for commercial achievement.
bioaccess® stands out by delivering ethical approvals in 4-6 weeks and achieving enrollment 50% faster than traditional markets, further supporting its clients in navigating these complexities.
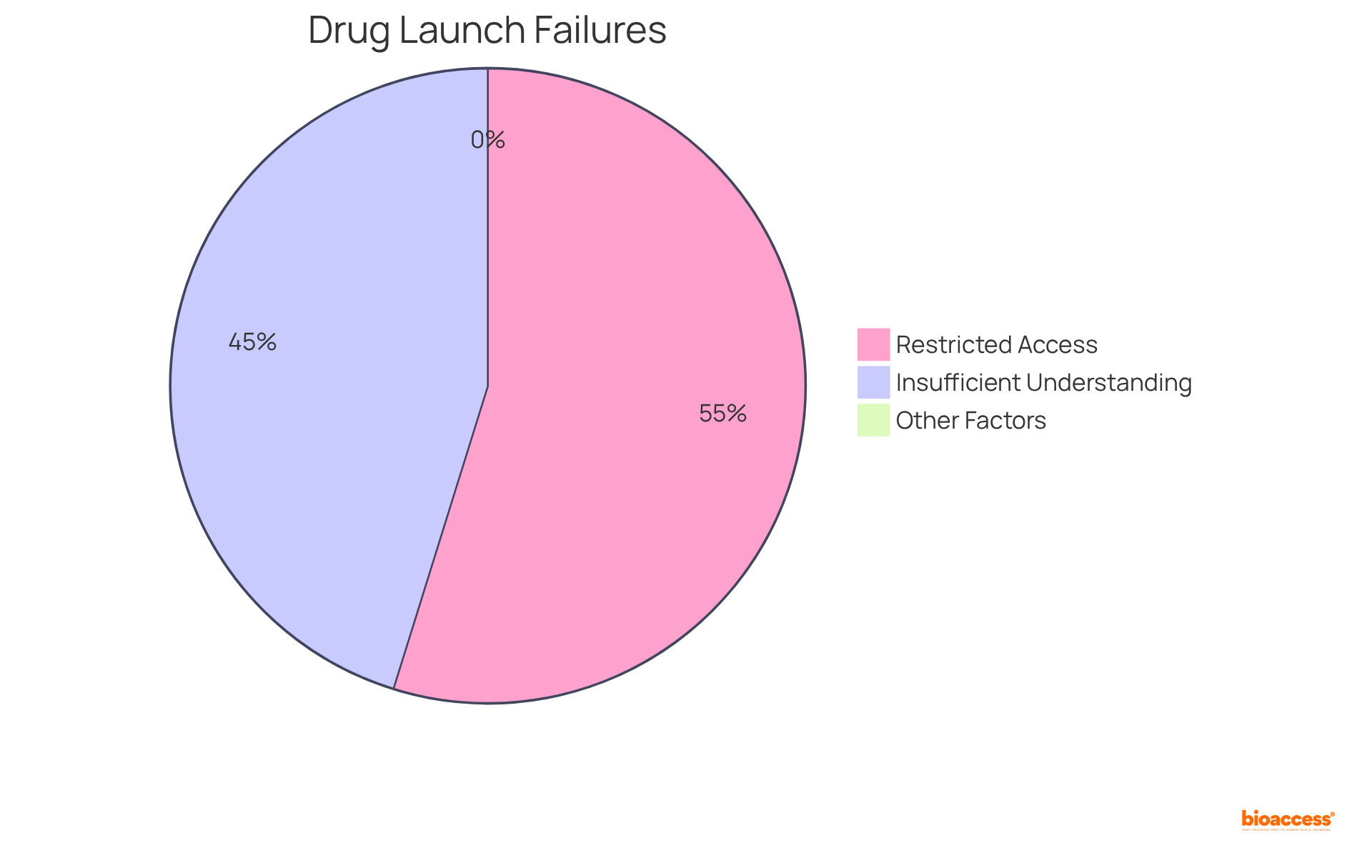
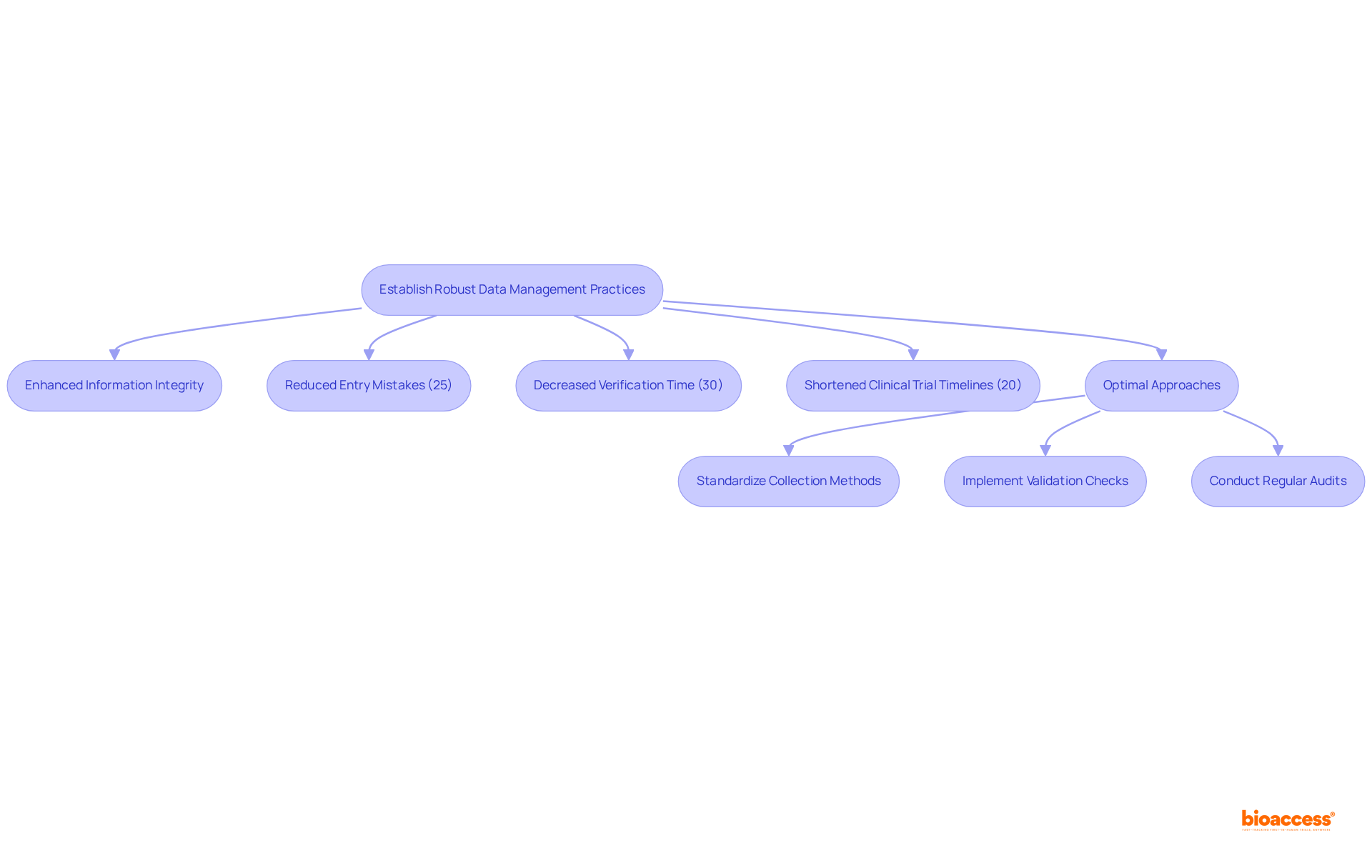
Establishing robust information management practices is crucial for the successful formulation drug process. Utilizing electronic information capture (EDC) systems significantly enhances information integrity and optimizes processes. EDC systems facilitate precise information collection, storage, and analysis, enabling real-time decision-making essential in clinical research. Research has demonstrated that EDC systems can reduce entry mistakes by 25% compared to conventional paper techniques, thereby enhancing overall information quality. Additionally, EDC systems can decrease information collection and verification time by 30%, further improving efficiency.
Moreover, EDC systems assist in adhering to regulatory standards, including 21 CFR Part 11 and GDPR, ensuring that privacy and integrity are maintained throughout the clinical trial process. Optimal approaches for utilizing EDC involve:
The impact of EDC systems on the efficiency of formulation drug processes cannot be overstated. By optimizing information management, these systems can shorten clinical trial timelines by as much as 20%, allowing quicker market access for new therapies. As the sector transitions towards decentralized and patient-focused trials, with an anticipated compound annual growth rate (CAGR) of 30.1% from 2021 to 2026, the significance of EDC systems in enhancing patient involvement and adherence to entry requirements becomes increasingly evident. Ultimately, prioritizing strong data management through EDC systems not only enhances the development process but also propels the advancement of medical innovations. As Mr. Hiren Thakkar states, "The potential of technology in clinical research is immense, and leveraging it effectively can lead to significant improvements in outcomes.
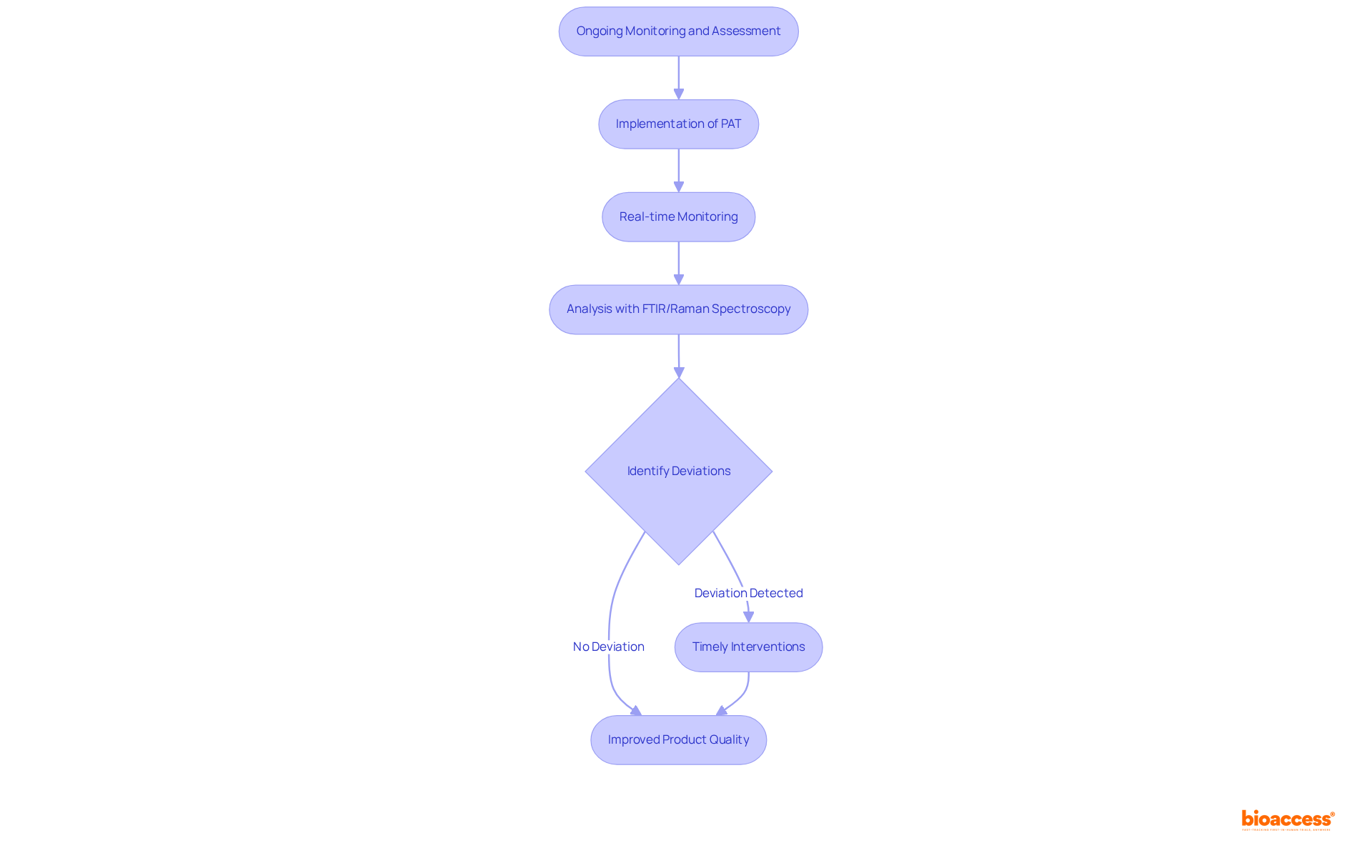
Ongoing monitoring and assessment of development processes are essential for ensuring quality and regulatory compliance in pharmaceutical manufacturing. The implementation of Process Analytical Technology (PAT) enables real-time monitoring of critical parameters, empowering manufacturers to track variations and maintain control over production processes.
For instance, tools like in-situ FTIR and Raman spectroscopy allow for immediate analysis of critical quality attributes (CQAs), significantly enhancing the understanding of reaction dynamics and product quality. Regular evaluations are vital in identifying deviations from expected outcomes, facilitating timely interventions that prevent large-scale production issues.
This proactive approach not only ensures that mixtures meet stringent regulatory standards but also optimizes resource utilization, minimizing waste and energy consumption. By adopting PAT, pharmaceutical companies can achieve a more efficient manufacturing process, ultimately leading to improved product quality and compliance with Good Manufacturing Practices (GMP).
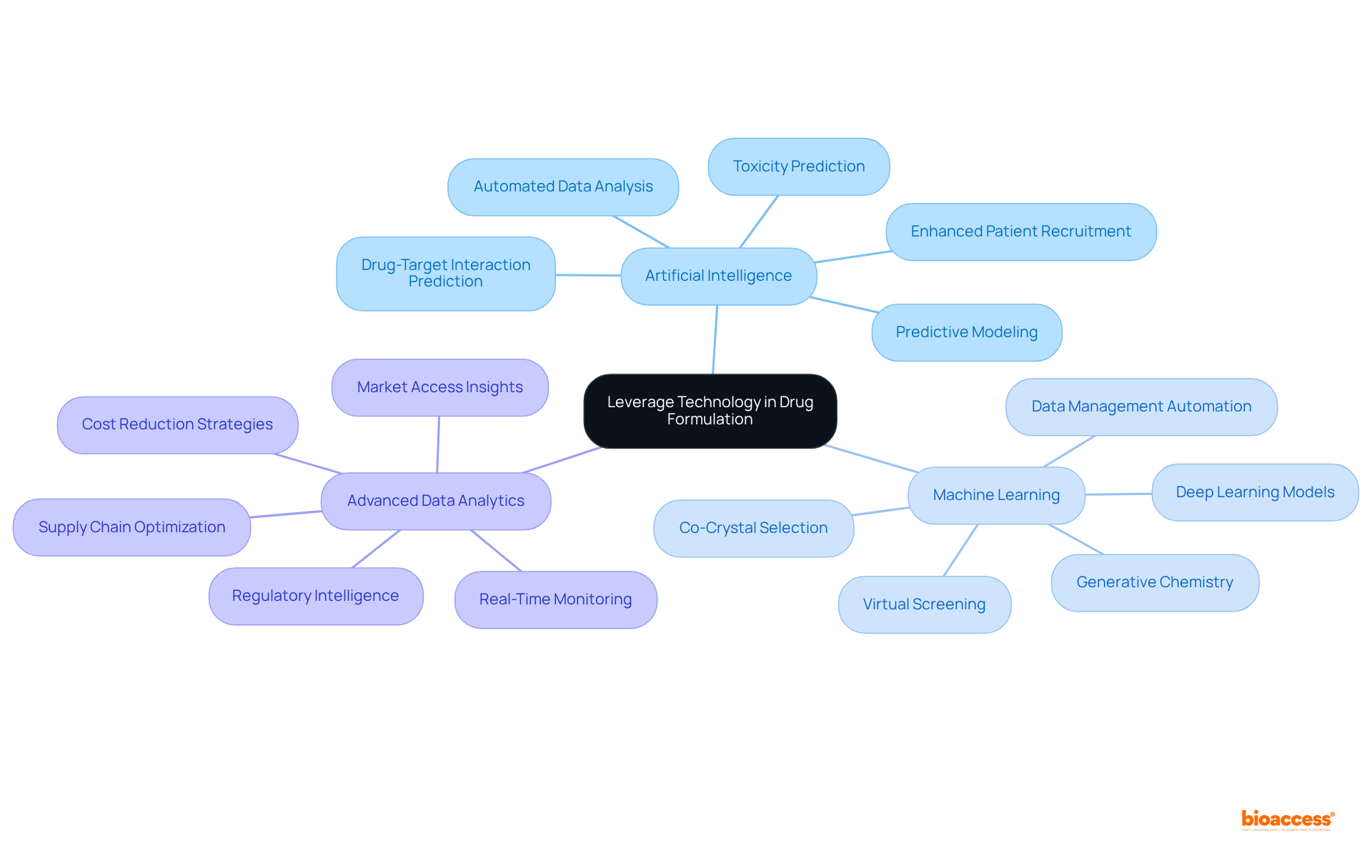
Utilizing technology is essential for enhancing the efficiency of formulation drug development. Innovations such as artificial intelligence (AI), machine learning, and advanced data analytics play a pivotal role in refining development processes. These technologies not only predict outcomes but also identify optimal conditions for success.
By embracing these advancements in formulation drug processes, companies can significantly shorten development timelines, reduce costs, and elevate the overall quality of their products. The integration of such technologies is not merely advantageous; it is imperative for staying competitive in the evolving landscape of clinical research.
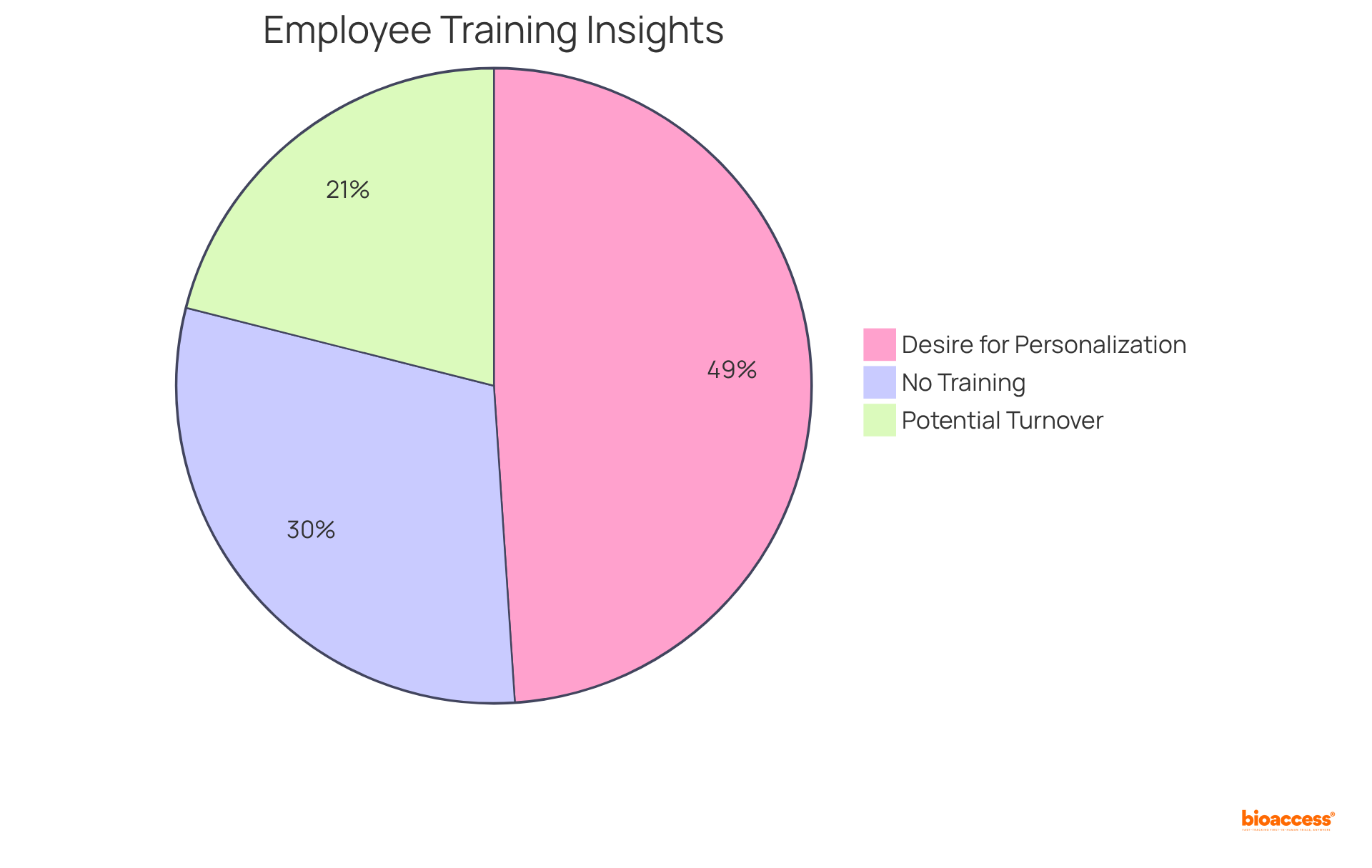
Investing in continuous education and training for development teams is crucial for fostering innovation and enhancing expertise within the pharmaceutical sector. Businesses must provide access to workshops, seminars, and online courses that focus on the latest advancements in medication preparation and regulatory requirements. Research indicates that organizations prioritizing team development experience a significant boost in performance, with training programs correlating to a 24% increase in workforce productivity. Furthermore, 93% of employees express a desire for personalized and relevant training, underscoring the necessity for tailored educational opportunities.
However, it is concerning that 57% of employees report having received no workplace training, highlighting the urgent need for companies to invest in training programs. Additionally, 40% of employees who do not receive essential job training are likely to leave within the first year, emphasizing the critical role of training in employee retention. Companies that have invested in comprehensive training initiatives have reported improved retention rates and employee satisfaction, with data showing that a robust training culture can reduce turnover and enhance career advancement opportunities.
By fostering a culture of continuous learning, organizations not only enhance their capabilities but also ensure they remain competitive in the rapidly evolving pharmaceutical landscape. As Imed Bouchrika, Co-Founder and Chief Data Scientist, stated, "Companies that do not invest in their employees are jeopardizing their own survival and success." This sentiment reflects the essential role that team development plays in the success of formulation drug efforts. Moreover, 70% of employees would consider leaving for a company that invests in their development, further illustrating the importance of training for employee retention.
The journey of successful drug formulation and development hinges on a multitude of strategic approaches that collectively enhance efficiency, compliance, and market readiness. This article emphasizes the critical nature of leveraging rapid clinical research services, understanding regulatory requirements, and fostering interdisciplinary collaboration to navigate the complexities of the pharmaceutical landscape effectively.
Key strategies outlined include:
Furthermore, the article highlights how technology plays a transformative role in streamlining drug development processes, while ongoing education and training for formulation teams are essential to maintaining a competitive edge in an ever-evolving field.
In conclusion, the significance of these strategies cannot be overstated. By embracing innovative practices, companies can not only expedite the development timeline but also enhance the quality and effectiveness of their products. As the pharmaceutical industry continues to evolve, the commitment to integrating these best practices will be paramount for achieving successful drug formulation and ensuring that new therapies reach patients in a timely and efficient manner.
What services does bioaccess® provide to accelerate drug development?
bioaccess® offers rapid clinical research services that significantly reduce the drug development timeline by leveraging regulatory speed in Latin America, diverse patient populations in the Balkans, and efficient ethical approval processes in Australia.
How quickly can bioaccess® achieve ethical approvals for clinical trials?
bioaccess® can achieve ethical approvals in just 4-6 weeks.
What advantages does bioaccess® provide in patient enrollment rates?
Patient enrollment rates through bioaccess® are 50% faster than traditional markets, which is beneficial for Medtech, Biopharma, and Radiopharma innovators.
How does the FDA's acceptance of clinical trial data from Mexican sites impact bioaccess®?
The FDA's acceptance reflects a growing recognition of the region's potential, and bioaccess® is positioned to facilitate compliance with Good Clinical Practice standards.
What are the financial benefits of conducting clinical research in Mexico?
Mexico's favorable exchange rates and lower operational costs allow U.S.-based companies to significantly reduce their clinical research budgets.
What role does bioaccess® play in mitigating delays in clinical studies?
bioaccess®'s rapid services help address the 30% of clinical studies that experience delays due to insufficient resource planning.
What are early-phase studies, and why are they important?
Early-phase studies, such as First-in-Human (FIH) and Early-Feasibility Studies (EFS), are essential for validating drug development strategies and gathering safety and efficacy data early on.
What percentage of drugs in FIH trials successfully progress to later phases?
Approximately 70% of drugs in FIH trials successfully progress to later phases.
How did bioaccess® support Avantec Vascular's trial in Latin America?
bioaccess® supported Avantec Vascular's first-in-human trial, resulting in a 50% increase in patient enrollment for subsequent phases due to robust safety data.
What challenges do early-phase studies face in participant recruitment?
Nearly 40% of first-in-human investigations fail to meet enrollment targets, highlighting significant challenges in participant recruitment.