


The article underscores the critical role of enhancing Randomization and Trial Supply Management (RTSM) in achieving clinical trial success through a variety of strategic approaches. It highlights the necessity of integrating advanced technology, fostering patient engagement, and ensuring compliance to streamline processes. This integration is essential for reducing costs and improving efficiency in clinical studies, ultimately leading to timely and successful outcomes.
In the evolving Medtech landscape, Bioaccess plays a pivotal role in addressing the key challenges faced in clinical research. Collaboration among stakeholders is paramount, as it drives innovation and enhances the overall effectiveness of clinical trials.
As we move forward, it is crucial to consider how these strategies can be implemented to optimize trial management and achieve superior results.
In the realm of clinical trials, the pressure to accelerate timelines while maintaining compliance and efficiency is ever-increasing. Innovative Randomization and Trial Supply Management (RTSM) solutions are at the forefront, enabling organizations to significantly enhance their study outcomes and operational agility. However, as the landscape evolves, research teams face the challenge of navigating the complexities of RTSM to ensure success. This article delves into nine strategic approaches designed to streamline processes, improve patient engagement, and ultimately drive clinical trial success.
bioaccess® harnesses cutting-edge solutions to significantly shorten clinical study timelines. By integrating advanced technology with comprehensive local regulatory expertise, bioaccess® guarantees that studies are both compliant and efficient. Their systems enhance patient randomization and streamline supply chain coordination, facilitating quicker decision-making and increased operational agility. This capability is particularly beneficial in the fast-paced environments of Medtech and Biopharma, where minimizing time-to-market is crucial.
With over 20 years of experience in Medtech, bioaccess® specializes in managing:
This ensures comprehensive clinical management services. Recent advancements in rtsm technology, including real-time analytics and automated supply tracking, have led to notable improvements in efficiency. Research indicates that rtsm tools can reduce testing durations by 20-30%, and assessments that utilize rtsm can conclude more swiftly and achieve enhanced data precision.
Moreover, the integration of remote management systems with patient-centric approaches has been linked to improved enrollment and retention rates, addressing common challenges faced in contemporary research. By enrolling treatment-naive cardiology or neurology groups 50% faster than Western locations, bioaccess® not only accelerates studies but also achieves $25K savings per patient with FDA-ready data—eliminating rework and delays. To fully leverage the benefits of rtsm solutions, consider incorporating real-time analytics and automated monitoring in your research studies.

Bioaccess distinguishes itself in the RTSM software environment with robust features tailored to meet diverse study needs in RTSM. Notably, the platform's capability to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites represents a significant advancement, enabling sponsors to realize substantial cost savings of $25K per patient, all while providing FDA-ready data—no rework, no delays. This adaptability is crucial in today's fast-paced healthcare landscape, where responsiveness can dramatically impact outcomes.
Furthermore, bioaccess offers comprehensive services for managing studies, including:
These functionalities not only streamline the testing process but also effectively address common patient recruitment challenges faced by Medtech and biopharma startups.
Clinion emphasizes the critical role of strong forecasting in the oversight of clinical study supplies within the rtsm framework. By leveraging advanced analytics and rtsm, Clinion accurately predicts supply requirements based on enrollment rates and patient demographics. This proactive approach effectively mitigates the risks of stockouts and overstocking, ensuring that testing locations are equipped with the necessary materials when needed. Effective forecasting not only enhances operational efficiency but also leads to significant cost reductions, establishing rtsm as a vital component of successful project oversight.
As the healthcare research environment evolves in 2025, the integration of analytics and rtsm into supply chain oversight is increasingly crucial, reflecting current trends that prioritize data-informed decision-making and strategic resource distribution. In this context, bioaccess offers essential services for overseeing studies, including:
Collectively, these services facilitate effective supply chain administration, ensuring that research studies operate efficiently and comply with regulatory standards. To fully leverage the advantages of these services, research directors should prioritize the integration of robust forecasting methods into their supply chain rtsm strategies.
Veeva's RTSM systems exemplify adaptability, empowering sponsors to modify study protocols in response to emerging data using RTSM. This flexibility is essential in the dynamic landscape of clinical research, where rapid changes are commonplace. The platform facilitates real-time updates and adjustments, allowing testing teams to swiftly react to fresh insights or regulatory requirements.
In contrast, bioaccess® presents a unique advantage by enabling organizations to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, yielding substantial cost savings of $25K per patient with FDA-ready data—no rework, no delays. With the FDA's new mandate for diversity strategies in late-stage studies, bioaccess® is ideally positioned to assist organizations in navigating this evolving regulatory landscape.
The increasing acceptance of randomization and supply management in research and development, growing at an impressive rate of 22-23% annually, underscores the critical need for adaptability in RTSM. By prioritizing adaptability, bioaccess® empowers organizations to conduct experiments that are not only more efficient but also more effective, ultimately enhancing the likelihood of successful outcomes.
A case study featuring Reata Pharmaceuticals illustrates this principle, as their flexible system implementation allowed for rapid modifications in a complex study, showcasing the tangible benefits of the bioaccess® platform.
Patient retention serves as a cornerstone of successful research studies, significantly influencing the validity and reliability of study outcomes. As Michael Young asserts, "Patient retention is a critical aspect that directly impacts the success and validity of any study." Signant Health highlights that integrating patient-centric strategies into RTSM is essential for enhancing participant engagement. A notable example is the collaboration between GlobalCare Clinical Studies and bioaccess™, which has achieved an extraordinary reduction in clinical study participant recruitment time by over 50%, alongside participant retention rates exceeding 95% in Colombia.
Bioaccess™'s services, including mobile applications that provide reminders and educational materials, are pivotal in this approach, ensuring that patients remain informed and actively engaged throughout the study process. These tools not only facilitate improved communication but also tackle common dropout reasons by offering convenience and support.
By prioritizing the patient experience and employing effective retention strategies—such as personalized communication tailored to each patient and ongoing support—organizations can significantly enhance retention rates, leading to more robust and reliable research data. Furthermore, addressing the financial implications of high dropout rates is vital, as these can result in increased costs and delays in drug approvals. Ultimately, these strategies are instrumental in the overall success of medical studies.
On March 29, 2019, bioaccess™ announced a strategic partnership with Caribbean Health Group, comprising eight prominent healthcare institutions in Barranquilla, Colombia. This collaboration aims to position the city as a premier destination for research in Latin America, an initiative supported by Colombia's Minister of Health. The objective is to enhance the research landscape by attracting more projects and participants.
By leveraging bioaccess®'s expert services, Medtech, Biopharma, and Radiopharma startups can achieve expedited research outcomes using rtsm. This includes over a 50% reduction in recruitment time and impressive 95% retention rates. Such a method addresses logistical challenges while aligning with the growing demand for patient-centered strategies in study design.
As the patient recruitment market for studies is projected to reach USD 1.57 billion by 2030, these strategies are vital for sponsors looking to elevate their research efforts. The partnership not only underscores the importance of collaboration but also sets the stage for transformative advancements in clinical research.
Bioaccess prioritizes compliance and regulatory adherence within its RTSM solutions, recognizing that these aspects are essential for the success of research studies in 2025. By ensuring that all systems meet stringent regulatory requirements, Bioaccess effectively mitigates risks associated with non-compliance. Their strategy encompasses:
This approach provides sponsors with peace of mind. Such steadfast dedication to adherence not only protects patient safety but also enhances the reliability of study outcomes, ultimately contributing to increased success rates in research. As noted by industry experts, adherence to regulatory standards is crucial for maintaining the integrity of clinical research and fostering trust among stakeholders.
Moreover, statistics reveal that 87% of organizations report negative results due to low compliance maturity, underscoring the critical nature of robust RTSM solutions for success in research. Integrating automated audit trails and real-time inventory monitoring can further bolster compliance initiatives, ensuring that assessments are conducted efficiently and effectively. To maximize the benefits of compliance, sponsors should regularly review and update their compliance strategies in line with regulatory changes.
Almac Group emphasizes the enhancement of inventory oversight to guarantee smooth clinical study operations. Their approach includes the implementation of rtsm, which provides continuous visibility into supply status and location. This proactive management extends to monitoring expiration dates, allowing for timely replenishment and minimizing waste. By adopting just-in-time inventory practices, Almac effectively reduces excess stock and ensures that testing locations are equipped with the necessary materials exactly when required. This strategic efficiency not only lowers operational costs but also significantly enhances the overall success rates of clinical trials.
As noted, streamlining the handling of study supplies is crucial to reduce waste and enhance efficiency. A case study from a large academic medical center showed that implementing an automated inventory oversight system led to a 50% decrease in inventory-related errors, highlighting the tangible advantages of effective inventory practices. Additionally, with 90% of clinical trials not succeeding due to various factors, optimizing inventory oversight becomes crucial for enhancing trial results.
To further enhance inventory management, organizations should consider implementing an rtsm, which can provide real-time tracking and improve overall efficiency.
Bioaccess underscores the significance of comprehensive training and support for users of their RTSM. By implementing tailored training programs, they ensure that all stakeholders—from site staff to sponsors—are equipped to effectively utilize the rtsm system. This educational emphasis not only reduces mistakes but also greatly improves the overall effectiveness of operational procedures. Moreover, continuous support, including 24/7 customer assistance and access to documentation, enables users to skillfully handle challenges that may occur during the testing process, ensuring a smoother operational flow.
With bioaccess's extensive services—including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting—the integration of these elements ensures that studies are not only expedited but also aligned with regulatory requirements. As observed, 'Education is the key that unlocks the golden door to freedom,' emphasizing the transformative influence of effective training in research operations.
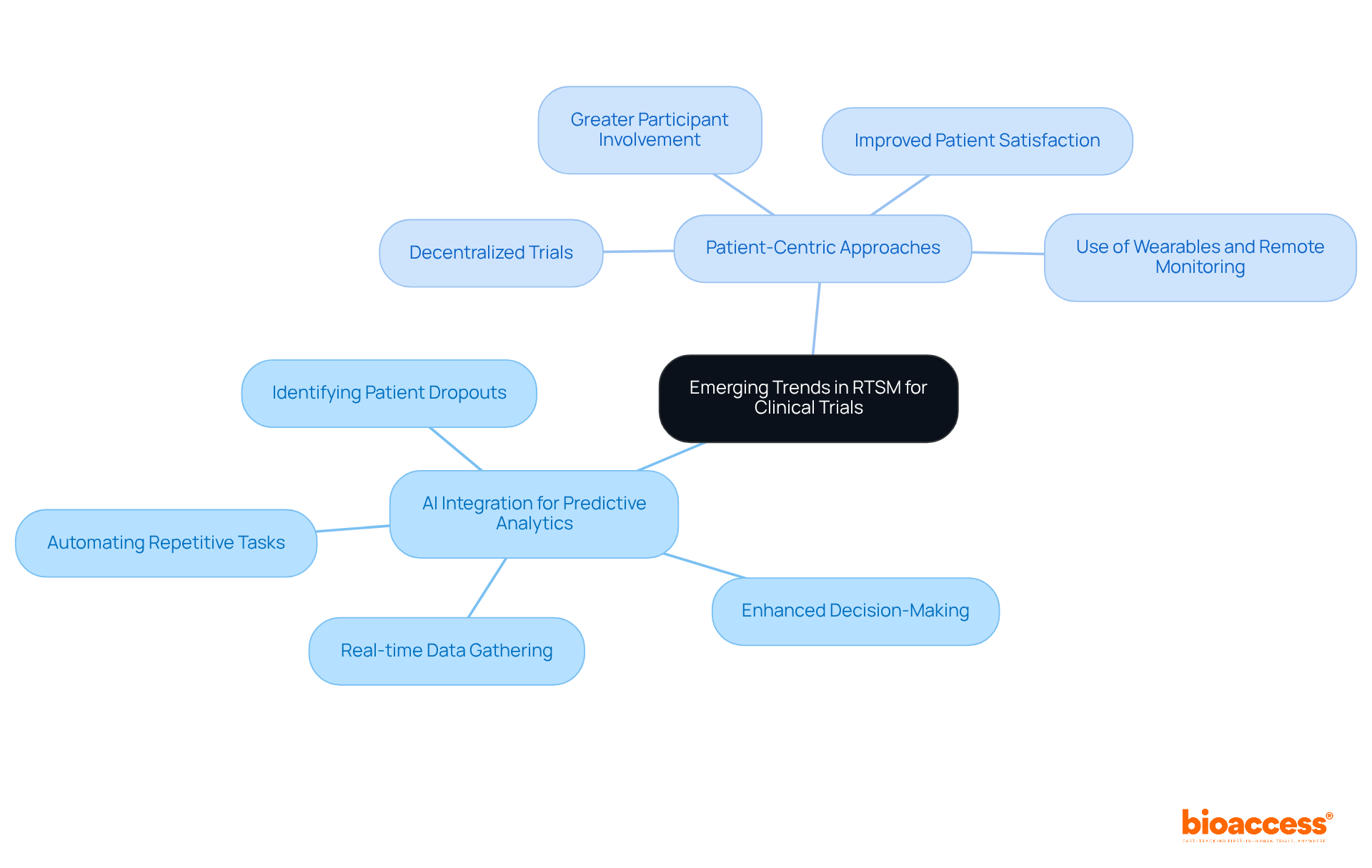
Xtalks identifies several emerging trends in RTSM that are poised to greatly impact research success. A key focus is the integration of artificial intelligence (AI) for predictive analytics, enhancing decision-making by analyzing vast datasets to identify patterns and predict outcomes. This capability is especially valuable in decentralized studies, where real-time data gathering and patient involvement are essential.
Additionally, the shift towards patient-centric approaches is becoming increasingly important, fostering greater participant involvement and satisfaction. As the clinical trial landscape evolves, sponsors must remain vigilant and adapt their strategies to leverage RTSM advancements, ensuring improved trial outcomes and operational efficiencies.
The strategies outlined for enhancing Randomization and Trial Supply Management (RTSM) are pivotal for achieving success in clinical trials. By leveraging innovative technologies and approaches, organizations can streamline processes, improve patient engagement, and ensure regulatory compliance, ultimately leading to faster and more efficient study outcomes.
Key insights from the article highlight the importance of integrating advanced analytics, patient-centric strategies, and robust forecasting methods into RTSM frameworks. Companies like bioaccess® and Clinion demonstrate how tailored solutions can significantly reduce recruitment times and operational costs while maintaining high retention rates. Furthermore, the emphasis on compliance and comprehensive training underscores the necessity of a well-rounded approach to RTSM that addresses both logistical and regulatory challenges.
As the clinical research landscape continues to evolve, it is essential for stakeholders to adopt these strategies and stay informed about emerging trends. Embracing advancements in technology and prioritizing patient engagement not only enhances trial success but also fosters trust and collaboration within the healthcare community. By implementing these best practices, organizations can position themselves at the forefront of clinical trial innovation, ultimately improving patient outcomes and accelerating the path to market for new therapies.
What is bioaccess® and what does it offer for clinical trials?
bioaccess® is a provider that harnesses innovative RTSM (Randomization and Trial Supply Management) solutions to significantly shorten clinical study timelines. They integrate advanced technology with local regulatory expertise to ensure compliance and efficiency in clinical trials.
What types of studies does bioaccess® manage?
bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.
How does bioaccess® improve clinical trial efficiency?
Recent advancements in RTSM technology, such as real-time analytics and automated supply tracking, have improved efficiency, reducing testing durations by 20-30% and enhancing data precision.
What benefits does bioaccess® provide in terms of patient enrollment?
The integration of remote management systems with patient-centric approaches has led to improved enrollment and retention rates, enabling bioaccess® to enroll treatment-naive cardiology or neurology groups 50% faster than Western locations.
What are the cost savings associated with using bioaccess®?
bioaccess® achieves savings of $25K per patient by providing FDA-ready data, eliminating rework and delays in clinical trials.
What additional services does bioaccess® offer for managing studies?
bioaccess® provides comprehensive services including feasibility assessments, site selection, compliance evaluations, setup, import permits, project coordination, and reporting.
How does Clinion contribute to supply chain management in clinical trials?
Clinion emphasizes the importance of strong forecasting in managing clinical study supplies within the RTSM framework, using advanced analytics to predict supply requirements and mitigate risks of stockouts and overstocking.
Why is effective forecasting important in clinical trials?
Effective forecasting enhances operational efficiency and leads to significant cost reductions, making RTSM a vital component of successful project oversight.
What are the current trends in supply chain oversight for clinical studies?
The integration of analytics and RTSM into supply chain oversight is increasingly important, reflecting trends that prioritize data-informed decision-making and strategic resource distribution.
What should research directors prioritize for effective supply chain management?
Research directors should prioritize the integration of robust forecasting methods into their supply chain RTSM strategies to fully leverage the advantages of these services.