


Navigating the landscape of clinical research in Bosnia and Herzegovina demands a comprehensive grasp of the Clinical Ethics Committee (CEC) approval process. This step is crucial for upholding ethical standards in research. By mastering the intricacies of CEC requirements, researchers can gain significant advantages, ultimately enhancing participant protection and fostering trust in their studies.
However, the journey to obtaining this approval is often fraught with challenges, including documentation hurdles and lengthy review times. How can researchers effectively navigate these complexities to ensure swift and successful outcomes?
Clinical Ethics Committees (CECs) are vital independent entities responsible for reviewing research proposals to ensure they meet ethical standards. In Bosnia and Herzegovina, obtaining clinical ethics committee approval is a necessary step for any clinical trial involving human participants. The primary role of a CEC is to protect the rights, safety, and well-being of research subjects by thoroughly assessing the ethical implications of proposed studies. This includes a detailed evaluation of the risk-benefit ratio, ensuring robust informed consent procedures, and confirming compliance with both national and international ethical guidelines, such as the Declaration of Helsinki.
The significance of CECs extends beyond mere regulatory compliance; they are essential in fostering trust within the research framework and enhancing participant protection. Successful case studies, like the one examining the 'Impact of Ethical Committees on Clinical Trials,' reveal that effective collaboration with CECs can streamline the authorization process, ultimately leading to quicker access to innovative treatments for patients. Furthermore, the ongoing discussions about establishing a unified ethical committee at the state level highlight the complexities and challenges faced in securing clinical ethics committee approval in Bosnia and Herzegovina.
Understanding the intricacies of the CEC's function is crucial for researchers aiming to navigate the endorsement landscape effectively while upholding the highest ethical standards in their work. As you consider your own challenges in clinical research, reflect on how collaboration with CECs can enhance your studies and protect your participants.

To successfully apply for clinical ethics committee approval in Bosnia and Herzegovina, it is essential to compile several key documents and pieces of information. These must meet the CEC's specific guidelines, ensuring a smooth application process. The following components are crucial:
It is crucial to ensure that all documents are complete, accurate, and formatted according to the CEC's guidelines. Frequent documentation challenges in clinical trial submissions in Bosnia and Herzegovina often arise from outdated forms or incomplete entries, which can considerably prolong the authorization timeline. By adhering to these best practices, including the use of clear and concise language in informed consent forms, researchers can facilitate a smoother review process and enhance the likelihood of obtaining clinical ethics committee approval in Bosnia and Herzegovina.
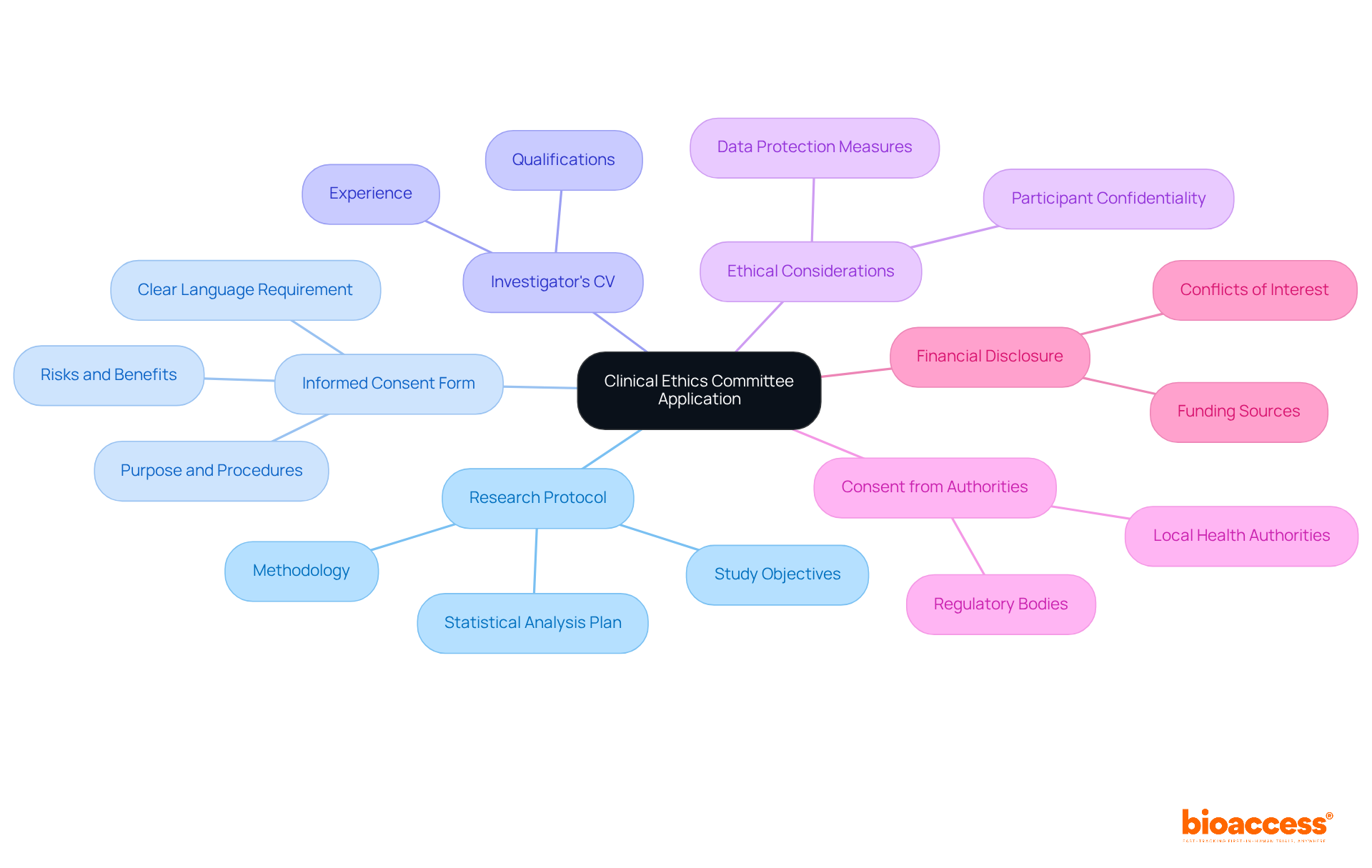
Once you have gathered all necessary documentation, the next step is to submit your request for clinical ethics committee approval in Bosnia and Herzegovina. This process is crucial for ensuring that your clinical research adheres to ethical standards and receives the necessary approvals. Follow these steps to enhance your chances of approval:
By adhering to these steps carefully, you can ensure that your application for clinical ethics committee approval in Bosnia and Herzegovina is submitted accurately and effectively, enhancing the chances of a prompt acceptance. Remember, the bureaucratic registration procedure for clinical trials can take several months to years, so establishing realistic expectations is crucial.

Navigating the clinical ethics committee approval in Bosnia and Herzegovina process is crucial for successful clinical research. However, it can be fraught with challenges. Here are key issues and effective strategies to overcome them:
Incomplete Documentation: Submitting a complete request is essential. Ensure that all required documents align with the CEC's checklist to prevent unnecessary delays. The registration process can take up to 1.5 years due to bureaucratic hurdles, making thorough documentation vital. Bioaccess provides comprehensive support in reviewing and providing feedback on study documents to ensure compliance with local requirements.
Lengthy Review Times: The timeline for CEC reviews can vary significantly, often ranging from 1 to 15 months. If your submission is delayed, proactively follow up with the CEC to check on its status. Maintaining a polite and professional tone in your communications fosters a positive relationship. Bioaccess can assist in project management and monitoring to effectively track these timelines.
Feedback and Revisions: Be prepared for constructive criticism from the CEC, which may require changes to your submission. Address all comments thoroughly and resubmit your request quickly to keep things progressing. Bioaccess's expertise in trial setup and management can help streamline this process.
Regulatory Changes: Staying informed about local regulatory changes that could affect your request is vital. Consistently check with the CEC or relevant authorities for the latest information, as these changes can influence timelines and requirements. Bioaccess is well-versed in navigating these regulatory landscapes, ensuring that your application remains compliant.
Communication Barriers: Language and cultural differences can complicate the validation process. Engaging a local expert or consultant who understands the CEC's procedures can facilitate smoother communication. Bioaccess can provide local insights and support to enhance communication with the CEC.
By anticipating these challenges and implementing these strategies, you can significantly improve your chances of obtaining clinical ethics committee approval in Bosnia and Herzegovina.

Achieving clinical ethics committee approval in Bosnia and Herzegovina is a crucial step for researchers conducting clinical trials involving human participants. This process not only ensures compliance with ethical standards but also fosters trust and enhances the protection of research subjects. By understanding the role of Clinical Ethics Committees (CECs) and navigating the approval process effectively, researchers can contribute to the advancement of medical knowledge while upholding the highest ethical standards.
The article outlines essential steps, including gathering required documentation such as:
It highlights the importance of thoroughness in submissions to avoid common pitfalls like incomplete documentation and lengthy review times. Additionally, strategies for overcoming challenges, such as:
are emphasized to facilitate a smoother approval process.
In conclusion, while the path to obtaining clinical ethics committee approval may be complex, it is vital for ensuring ethical integrity in clinical research. Researchers are encouraged to approach this process with diligence and a proactive mindset, recognizing the pivotal role that ethics committees play in safeguarding participant welfare and advancing medical research. By adhering to best practices and seeking support when needed, researchers can navigate the approval landscape successfully, ultimately leading to innovative treatments and improved patient outcomes.
What is the role of Clinical Ethics Committees (CECs)?
CECs are independent entities responsible for reviewing research proposals to ensure they meet ethical standards, protecting the rights, safety, and well-being of research subjects.
Why is obtaining CEC approval necessary in Bosnia and Herzegovina?
CEC approval is necessary for any clinical trial involving human participants to ensure ethical compliance and protect the rights and safety of those involved.
What aspects do CECs assess in research proposals?
CECs evaluate the ethical implications of proposed studies, including the risk-benefit ratio, informed consent procedures, and compliance with national and international ethical guidelines.
How do CECs contribute to participant protection and trust in research?
CECs foster trust within the research framework and enhance participant protection by ensuring that research proposals adhere to ethical standards.
What is an example of a successful collaboration with a CEC?
The case study on the 'Impact of Ethical Committees on Clinical Trials' demonstrates that effective collaboration with CECs can streamline the authorization process and lead to quicker access to innovative treatments.
What are the current discussions regarding CECs in Bosnia and Herzegovina?
There are ongoing discussions about establishing a unified ethical committee at the state level, highlighting the complexities and challenges in securing CEC approval.
Why is it important for researchers to understand the function of CECs?
Understanding the intricacies of CECs is crucial for researchers to navigate the endorsement landscape effectively while upholding the highest ethical standards in their work.