


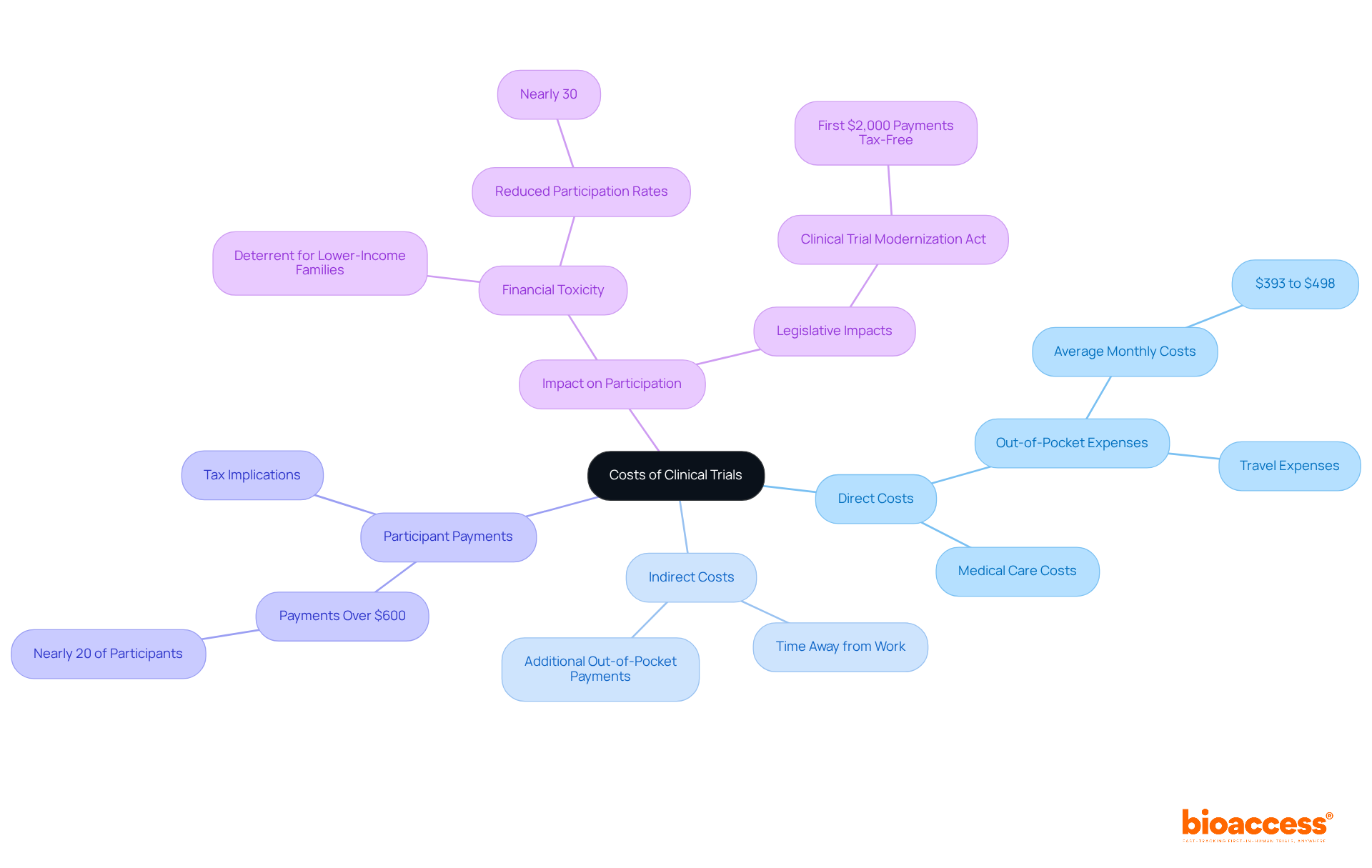
Clinical trials present challenges for participants, as they often face significant out-of-pocket expenses, including travel, meals, and lost income. While some studies do cover research-related medical care, the article underscores that these indirect costs can accumulate to hundreds of dollars each month. This financial burden can deter participation, particularly among lower-income individuals. Therefore, it is crucial to ensure transparency regarding costs and compensation in clinical studies. Addressing these issues not only fosters trust but also encourages broader participation in vital clinical research.
Understanding the financial landscape of clinical trials is crucial for potential participants. Many individuals are left wondering whether their involvement incurs any costs. While certain medical care related to research may be covered, participants frequently face unexpected expenses that can significantly affect their financial situation. In fact, nearly 30% of individuals express uncertainty about the costs associated with clinical trials. This raises an important question: are clinical trials genuinely free, or do hidden costs lurk beneath the surface?
Exploring this complex issue reveals critical insights into:
By delving into these aspects, we can better understand the true cost of participation and empower individuals to make informed decisions.
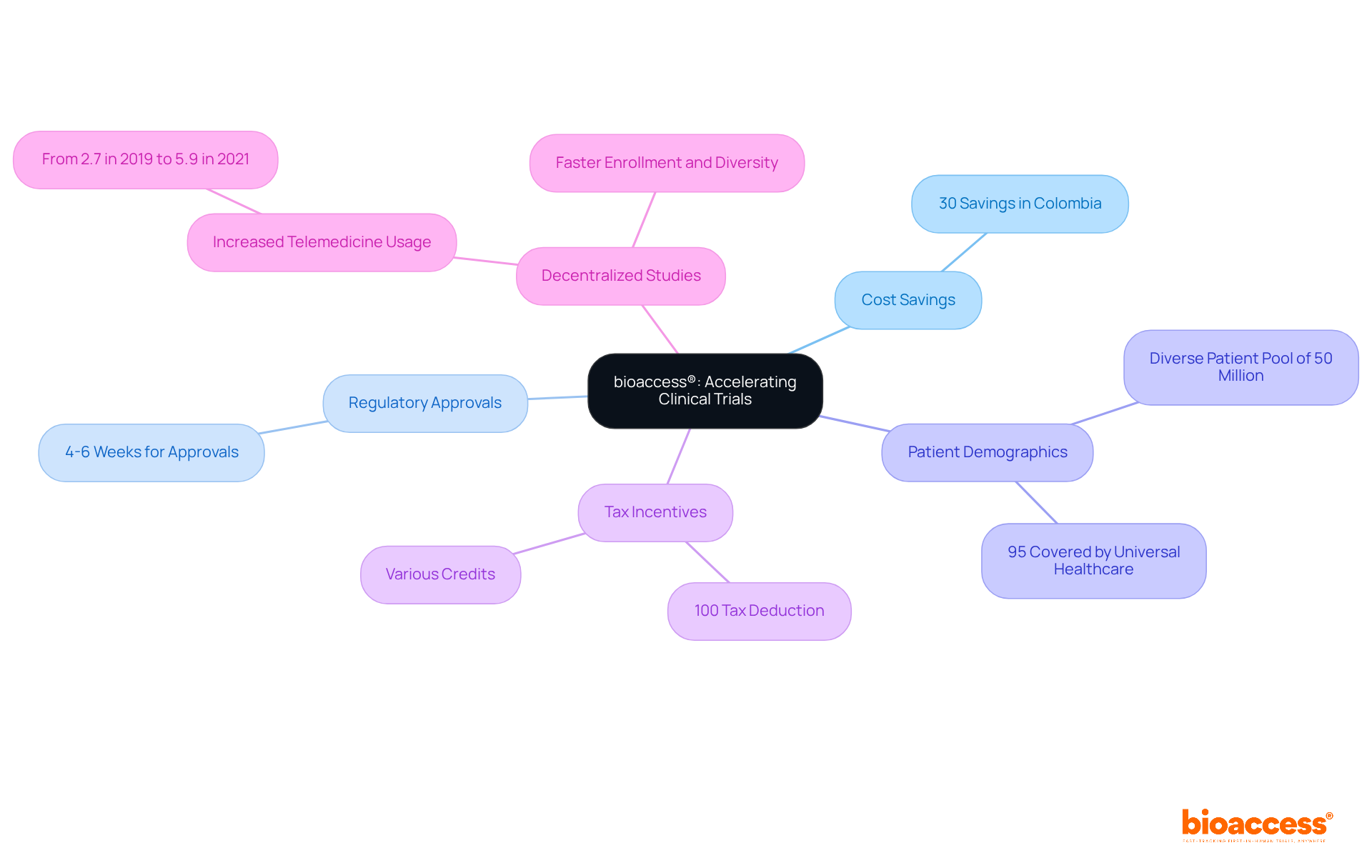
bioaccess® leverages its extensive expertise across Latin America, the Balkans, and Australia to deliver innovative solutions that significantly cut down the time and costs associated with research studies. In Colombia, research studies can achieve savings exceeding 30% compared to North America and Western Europe, with regulatory approvals expedited to just 4-6 weeks. By tapping into Colombia's diverse patient demographic of over 50 million—where approximately 95% are covered by universal healthcare—bioaccess® accelerates the research process and enhances cost-effectiveness. This strategy alleviates financial pressures for stakeholders, as patient recruitment costs account for about 40% of the total budget, amounting to around $1.89 billion, while also facilitating quicker access to groundbreaking treatments.
Moreover, Colombia offers substantial R&D tax incentives, including a 100% tax deduction and various credits, making it an attractive destination for research studies. Recent advancements in decentralized studies, particularly the increase in telemedicine usage during Phase III evaluations—from 2.7% in 2019 to 5.9% in 2021—further bolster these initiatives, enabling faster enrollment and greater participant diversity, which are essential for achieving recruitment targets. As a result, bioaccess® is at the forefront of transforming research, ensuring that the transition from innovation to patient care is both efficient and economically sustainable. The FDA's guidance on decentralized studies underscores the regulatory support for these innovative approaches, reinforcing bioaccess®'s commitment to advancing medical research.
Individuals often wonder if clinical trials are free, as numerous clinical studies incorporate research-related medical care, yet they may still face significant indirect expenses that can impact their decision to participate. These costs typically include travel expenses, time away from work, and other out-of-pocket payments. For instance, recent research indicates that cancer study participants may incur average monthly out-of-pocket costs ranging from $393 to $498, primarily due to travel and related expenses. Additionally, nearly 20% of research study contributors reported receiving payments exceeding $600, which may lead to tax implications, as this income must be reported to the IRS.
Understanding the question of whether clinical trials are free is crucial, as expert opinions highlight their importance. Research shows that financial toxicity—defined as the economic burden of medical costs—can deter individuals from engaging in medical studies, particularly among lower-income families, who face barriers that make them question whether clinical trials are free, ultimately reducing participation rates by nearly 30%. The Clinical Trial Modernization Act aims to alleviate some of these financial burdens by ensuring that the first $2,000 in payments to participants are clinical trials free from taxation, thereby encouraging broader involvement.
The financial consequences of clinical study participation are evident in case studies examining associated costs. For example, individuals in oncology studies often incur higher expenses than those receiving standard treatment, with some research suggesting that study participants may spend nearly $500 more each month. This disparity underscores the need for transparency and support from study sponsors to clarify whether clinical trials are free to mitigate these costs.
In light of recent developments, including proposed reductions to indirect research expenses by the NIH, the funding landscape for medical studies remains uncertain. Institutions like the University of Pittsburgh have successfully organized to maintain high indirect cost recovery rates, which are essential for supporting medical research and ensuring that financial barriers do not hinder patient access to experimental studies. Joan Gabel, Chancellor of the University of Pittsburgh, has warned that such funding cuts could cause 'irreparable harm' to research patients and university operations. Understanding these dynamics is vital for prospective participants as they navigate the complexities of determining if clinical trials are free.
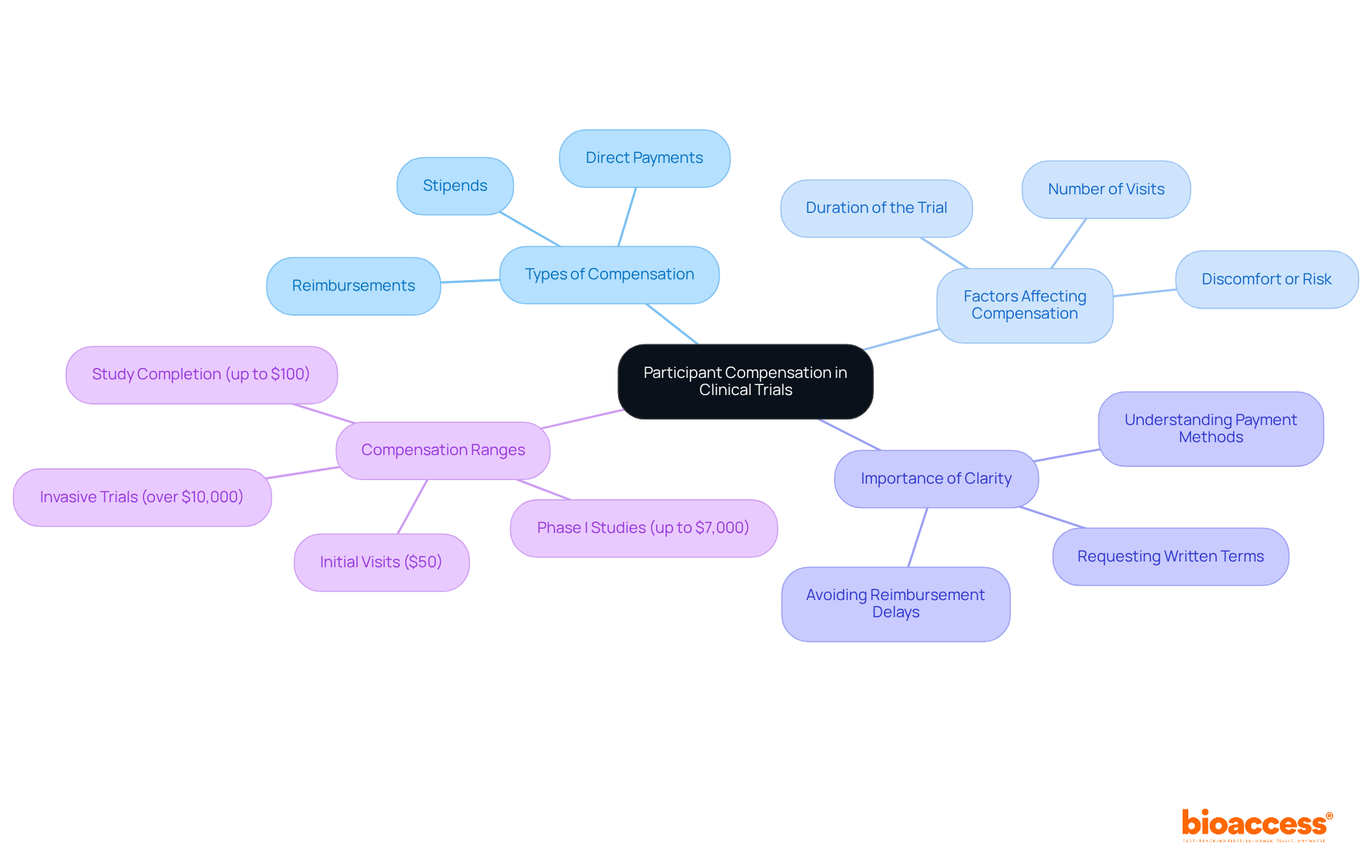
Participants in clinical studies often receive compensation for their time and the inconveniences related to their involvement. This compensation framework can vary significantly based on the complexity, duration, and specific requirements of the assessment. For instance, some studies may offer stipends, while others provide reimbursement for travel and associated costs. Typically, compensation for completing initial visits starts at around $50, with total payments for study completion reaching up to $100. More invasive trials, such as vaccine challenge studies or gene therapy pilots, can offer compensation exceeding $10,000, reflecting the increased effort and inconvenience for those involved. Additionally, Phase I studies with long inpatient stays may exceed $7,000 due to the considerable time and effort needed from subjects.
Compensation is typically prorated, meaning individuals receive payment according to their degree of involvement and the time commitment necessary. Factors affecting compensation include:
It is essential for individuals to clarify compensation details before joining a study. This includes understanding the payment methods, which can range from checks and direct deposits to cash cards or gift cards. Participants are encouraged to request a written schedule of events and payment terms from the trial organizers to avoid confusion.
Clarity in compensation practices is crucial, as it fosters trust and ensures individuals are fully aware of what to expect. Furthermore, individuals should be aware of possible reimbursement delays, which can lead to frustration and withdrawals. As Joseph Smith noted, "Reimbursement delays (even minor ones) often lead to frustration and withdrawals." It is important to note that individuals can exit the study at any time and still receive compensation for the duration of their involvement.
Overall, comprehending the compensation environment in research studies enables individuals to make informed choices about their involvement, ensuring they are fairly rewarded for their contributions.
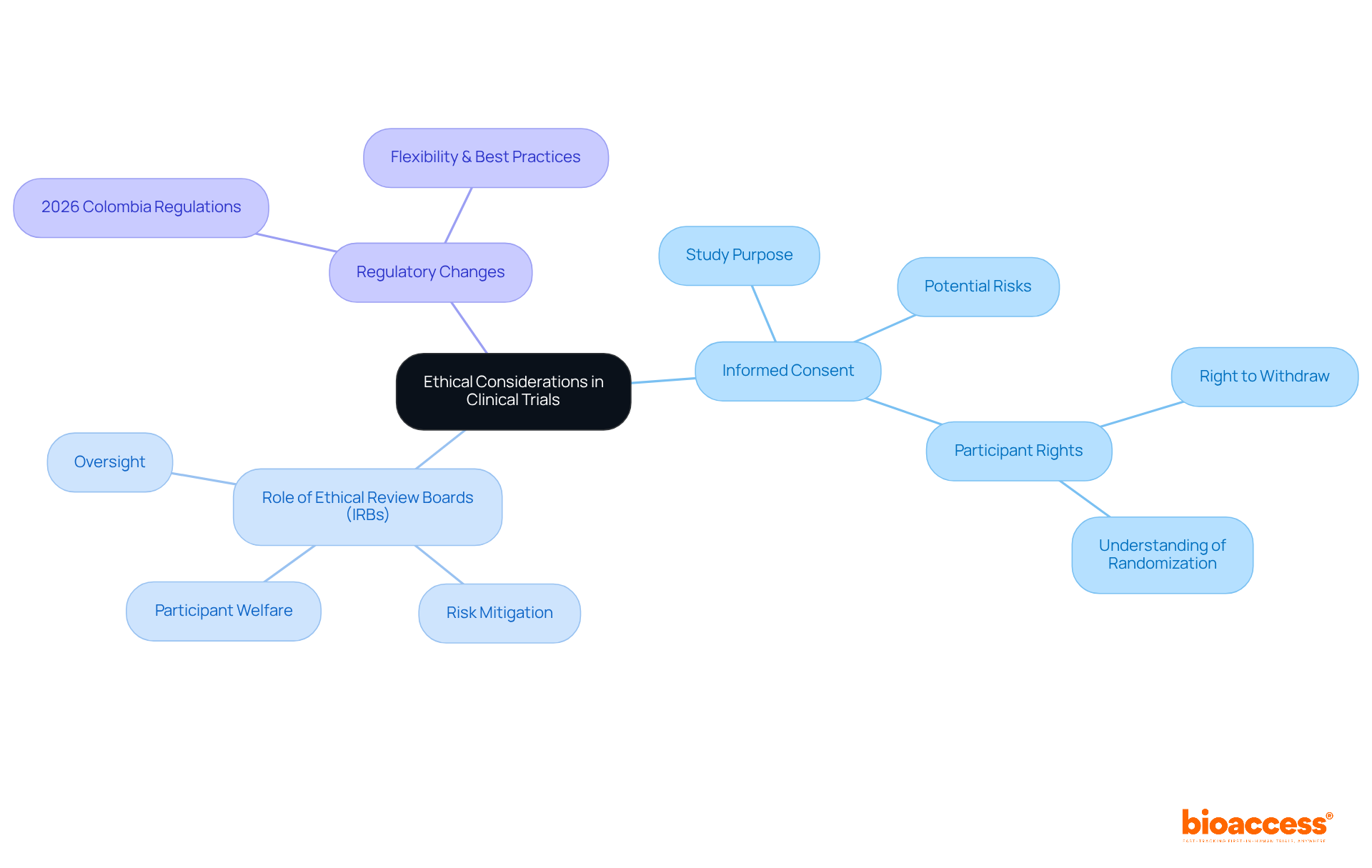
Ethical factors in clinical studies are vital for ensuring that individuals are treated with fairness and respect. A cornerstone of this ethical framework is the informed consent process, which must clearly communicate the study's purpose, potential risks, and benefits. Bioethicists emphasize that obtaining informed consent is essential for enhancing welfare and protecting individual rights. Alarmingly, only 75.8% of individuals recognize their right to withdraw from research studies, highlighting a significant gap in understanding that must be addressed to improve engagement and trust among participants.
Ethical review boards (IRBs) play a crucial role in overseeing research studies, ensuring adherence to ethical standards and safeguarding participant welfare. These boards evaluate study designs and methodologies, helping to mitigate risks and enhance the integrity of the research process. In 2023, serious events accounted for 4.1% of total reports in research trials, underscoring the importance of diligent oversight in ensuring participant safety.
As we approach 2025, the significance of ethics in medical research continues to grow, particularly with the evolving regulatory landscape. New medical research regulations set to be implemented in Colombia in April 2026 will promote flexibility and align with international best practices, further emphasizing the need for ethical vigilance in research. By fostering a culture of adherence and prioritizing the well-being of subjects, clinical research organizations can build trust and ensure the ethical treatment of individuals throughout the study process.
Clinical trials are organized into four distinct phases, each serving a specific purpose in the drug development process.
Understanding these phases is vital for participants to know if clinical trials are free, as each phase has unique goals and requirements. For instance, the typical length of research studies has increased, with FDA-approved medications averaging 89.8 months in evaluations from 2014 to 2018, compared to 83.1 months from 2008 to 2013. The expenses linked to these studies can be considerable; projections indicate that developing a new medication can range from $985 million to as much as $2.6 billion. This financial burden often arises from the high failure rates, with only 7.9% of drugs in Phase I ultimately receiving approval.
Recent discussions among medical researchers emphasize the importance of clear communication regarding study phases. Dr. Yvette Cozier noted that potential participants, particularly from marginalized groups, are eager to engage in research if the information is presented in an understandable manner. This underscores the necessity for transparency and knowledge about what each stage entails, facilitating informed choices for individuals considering participation in research studies. Moreover, the challenge of under-enrollment in research studies, especially among seniors, remains significant, highlighting the need for diverse involvement to ensure that medical advancements are relevant to all demographics.
At bioaccess, we provide comprehensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. Our expertise ensures that studies are conducted efficiently and in accordance with local regulations, contributing to the overall success of research initiatives. Furthermore, media coverage, including insights from Clinical Leader, highlights the growing interest in research studies in Latin America, particularly in Colombia, showcasing the region's potential for global partnership and innovation in Medtech.
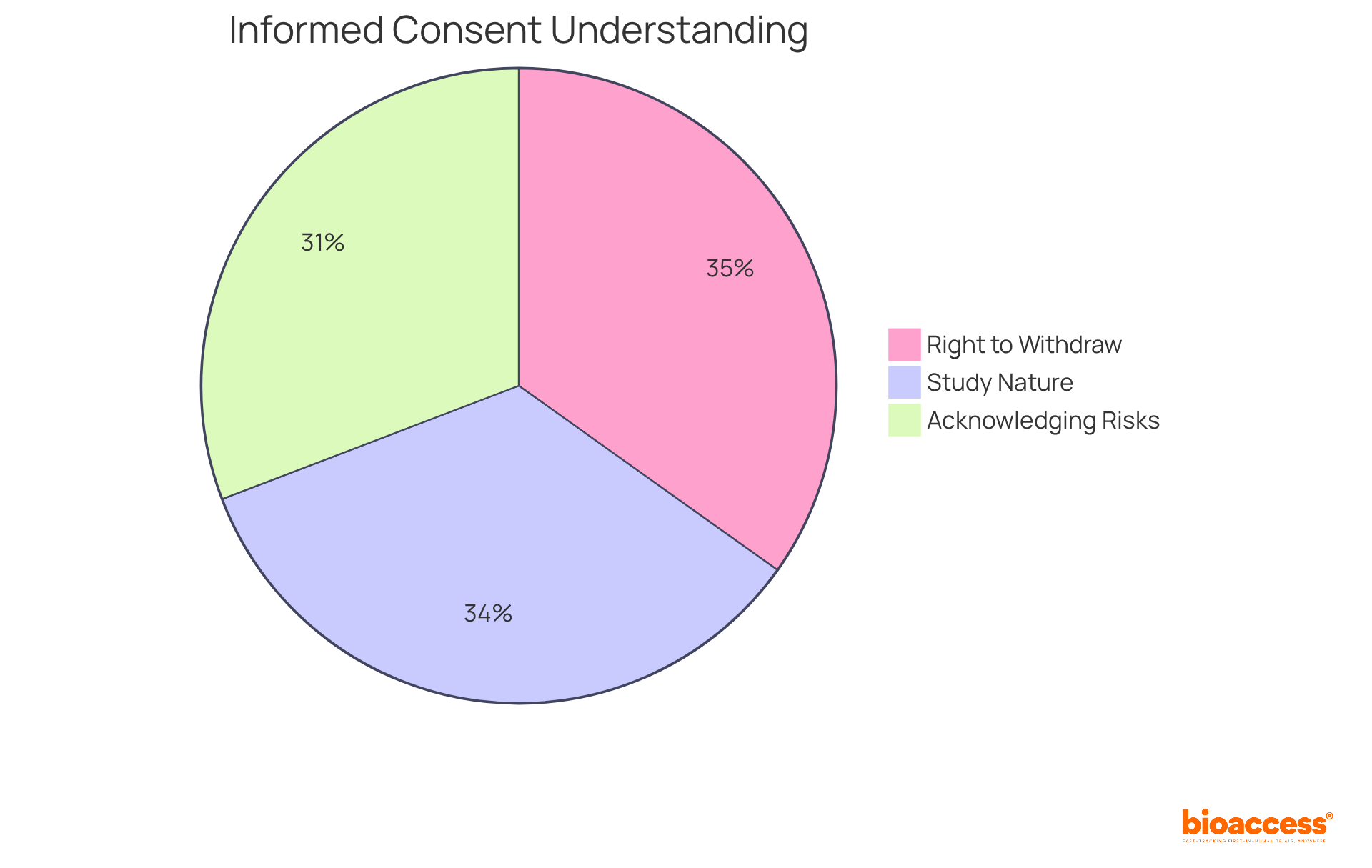
Informed consent stands as a cornerstone of clinical trials, ensuring individuals are fully aware of the study's purpose, procedures, risks, and benefits before agreeing to participate. This process transcends mere formality; it is vital for safeguarding individuals' rights and fostering trust between researchers and participants. Individuals possess the right to ask questions and must receive information that is clear and comprehensible, ideally presented at an eighth-grade reading level to enhance understanding.
Statistics reveal that while 74.7% of individuals grasp the nature of the study, only 67.0% acknowledge the potential risks and side effects involved. This underscores the necessity for researchers to communicate information effectively, as many individuals struggle with health literacy—nine out of ten adults in the U.S. face challenges in this domain. Furthermore, participants have the right to withdraw from a study at any time, a fact recognized by 75.8% of those engaged in trials.
The informed consent process should be viewed as an ongoing conversation rather than a one-time event, allowing individuals to voice concerns and ask questions throughout the study. By prioritizing transparency and clarity, researchers can enhance participants' understanding of their rights and responsibilities, ultimately leading to a more ethical and effective research environment.
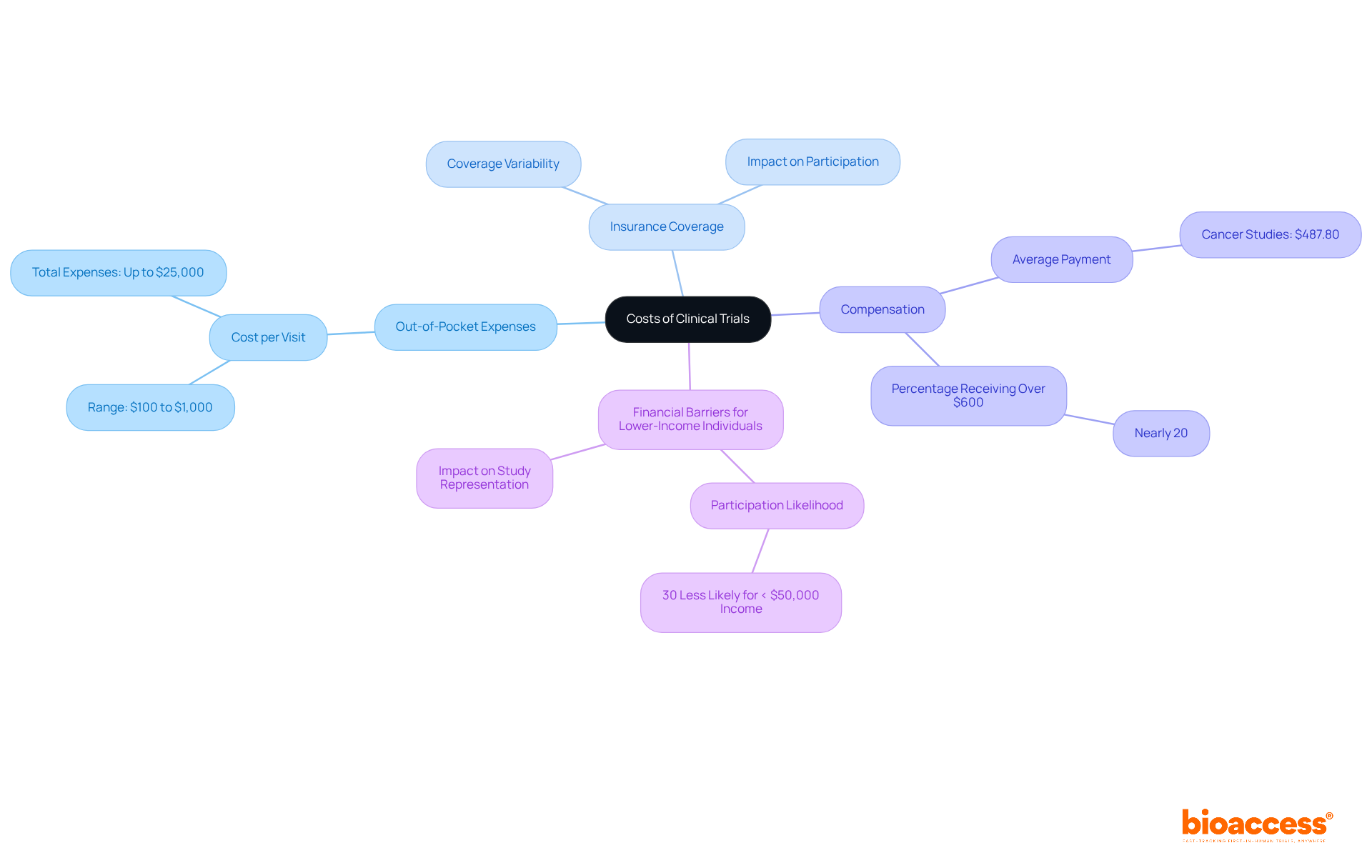
Insurance coverage for research study participation varies significantly among health plans. While many insurance companies cover routine healthcare expenses incurred during a legal process, this coverage is not guaranteed across the board. Approximately 29 states and the District of Columbia have enacted regulations requiring private insurers to cover research study costs; however, many plans still impose limitations. Notably, over 20% of patients denied coverage for research studies had insurance policies that explicitly excluded research study benefits.
The influence of insurance on clinical study participation is substantial. Financial barriers, such as high out-of-pocket costs for uncovered services, can deter eligible patients from joining studies. For instance, the median payment for patients in National Cancer Institute (NCI)-sponsored studies is around $750, compared to $2,500 for industry-sponsored studies, highlighting the disparities in coverage and reimbursement practices.
Experts emphasize the importance of understanding insurance agreements before participating in research studies. Justin F. Klamerus from the Sidney Kimmel Comprehensive Cancer Center points out that even insured patients may encounter significant obstacles due to exclusions in their coverage. He states, "Denial of entry to therapeutic research studies, even among insured patients, is a significant obstacle to cancer research."
Moreover, starting January 1, 2022, all state Medicaid programs are legally required to cover routine patient care expenses for participants in eligible research studies. The Affordable Care Act (ACA) mandates that new health insurance plans include standard expenses for participants in research studies related to life-threatening conditions. However, grandfathered plans, which existed before March 2010, are not obligated to provide such coverage, leaving many patients exposed to unexpected costs.
In conclusion, while insurance can alleviate some expenses associated with research participation, significant gaps remain. Participants should thoroughly review their insurance policies and consult with their providers to clarify coverage details, ensuring they are prepared for any potential out-of-pocket expenses during their involvement.
Participants in clinical studies often encounter a range of out-of-pocket costs that can significantly impact their financial health. These expenses typically include:
For instance, individuals living more than 100 miles from a treatment facility may experience heightened financial strain, with travel expenses alone potentially reaching up to $795 monthly. While some trials do offer compensation for these costs, a survey indicates that only 29% of participants received partial or full reimbursement for travel fees, highlighting the inconsistency in coverage.
Experts stress the necessity of discussing specific insurance policies with providers. As Kurnaz pointed out, patients should engage in serious conversations with their insurance providers regarding what their specific policy may or may not cover. Many patients report facing substantial financial burdens related to their participation; notably:
Understanding these financial implications is crucial for potential contributors, enabling them to make informed decisions about their involvement in research studies. It is advisable for individuals to thoroughly examine study documents to fully grasp the financial consequences before committing.

Diversity in clinical trials is crucial for ensuring that research findings resonate with a broader population. Trials that encompass a range of individuals yield more comprehensive data regarding treatment effectiveness and safety across various demographics. Research has shown that including a diverse array of participants can deepen our understanding of how different groups respond to therapies, which is vital for developing effective medical solutions.
Moreover, implementing diverse recruitment strategies can significantly reduce costs related to participant enrollment and retention. By engaging with marginalized communities and utilizing culturally sensitive approaches, researchers can foster trust and involvement, ultimately leading to more impactful studies. For instance, initiatives like community engagement and peer navigation models have proven effective in boosting enrollment among historically excluded groups, thereby enriching the participant pool.
The importance of diversity is further underscored by legislative measures such as the DEPICT Act, which mandates diversity action plans in research designs. This not only champions inclusivity but also guarantees that the resulting data is relevant to the intended user population, thereby enhancing the overall quality of research outcomes. As experts emphasize, embracing diversity in medical studies is not merely a recommendation; it is essential for advancing equitable healthcare and improving treatment effectiveness across all demographics.
It is crucial for potential participants to understand if clinical trials are free when navigating the expenses related to clinical studies. A clear understanding of various financial factors—such as out-of-pocket charges, insurance coverage, and compensation for those involved—can significantly impact decision-making. Individuals should actively seek clarity on whether clinical trials are free to ensure their choices align with their personal and financial circumstances. For instance, the typical out-of-pocket expense per visit can range from $100 to $1,000, with some participants reporting expenditures exceeding $25,000 during their involvement in studies.
Moreover, while reimbursement for participation averages around $487.80 in cancer studies, nearly 20% of participants received payments over $600. However, many individuals face substantial financial pressures, with 58% indicating that they are unsure if clinical trials are free of compensation for trial-related costs. Notably, those with household earnings below $50,000 annually are nearly 30% less likely to engage in research studies, which raises concerns about whether clinical trials are free for lower-income individuals, highlighting the financial barriers that can deter their participation. This results in less representative study populations.
By comprehending these dynamics, participants can navigate the financial landscape of research studies more effectively and advocate for their needs. As Jim Murphy emphasized, addressing known barriers to participation is essential for increasing both the number and diversity of individuals who volunteer for clinical trials.
Understanding the financial landscape of clinical trials is crucial for potential participants. Many people mistakenly believe that clinical trials are free, but the reality is that significant costs can arise from participation, including out-of-pocket expenses and indirect costs. By demystifying these financial factors, individuals can make informed decisions about their involvement in research studies, ensuring alignment with their personal and financial circumstances.
Key insights reveal that while some trials offer compensation, many participants still face substantial expenses, such as travel and lost income. These findings underscore the need for transparency and support from study sponsors to clarify the financial implications of participation. Furthermore, disparities in insurance coverage can create barriers for many individuals, particularly those from lower-income backgrounds, ultimately affecting diversity and representation in clinical research.
As the landscape of clinical trials continues to evolve, it is essential for prospective participants to actively seek information about their rights and responsibilities, as well as the financial aspects of their involvement. By advocating for clarity and understanding, individuals can help ensure that clinical research remains accessible and equitable, paving the way for advancements in healthcare that benefit all demographics.
What is bioaccess® and how does it benefit clinical trials?
bioaccess® is a company that leverages its expertise in Latin America, the Balkans, and Australia to provide innovative solutions that significantly reduce the time and costs of research studies. In Colombia, studies can achieve savings exceeding 30% compared to North America and Western Europe, with expedited regulatory approvals in just 4-6 weeks.
How does Colombia's healthcare system contribute to clinical trials?
Colombia has a diverse patient demographic of over 50 million people, with approximately 95% covered by universal healthcare. This facilitates quicker patient recruitment and enhances cost-effectiveness in clinical trials.
What are some financial incentives for conducting research in Colombia?
Colombia offers substantial R&D tax incentives, including a 100% tax deduction and various credits, making it an attractive destination for research studies.
How has telemedicine impacted clinical trials?
The use of telemedicine in decentralized studies has increased from 2.7% in 2019 to 5.9% in 2021, enabling faster enrollment and greater participant diversity, which are crucial for meeting recruitment targets.
Are clinical trials free for participants?
Clinical trials are not entirely free; while they may cover research-related medical care, participants often incur indirect costs such as travel expenses and time away from work. Recent studies show that cancer study participants may face monthly out-of-pocket costs ranging from $393 to $498.
What is financial toxicity and how does it affect participation in clinical trials?
Financial toxicity refers to the economic burden of medical costs, which can deter individuals, especially those from lower-income families, from participating in clinical trials. This can reduce participation rates by nearly 30%.
What compensation can participants expect in clinical trials?
Compensation for clinical trial participants varies based on the study's complexity and duration. Initial visit payments start at around $50, while total payments for study completion can reach up to $100. More invasive trials may offer compensation exceeding $10,000.
How is participant compensation structured in clinical trials?
Compensation is typically prorated based on the participant's involvement and time commitment. Factors affecting compensation include the duration of the study, the number of visits, and any discomfort or risk associated with the procedures.
What should participants clarify regarding compensation before joining a study?
Participants should understand the payment methods and request a written schedule of events and payment terms from trial organizers to avoid confusion.
Can participants withdraw from a study, and will they still receive compensation?
Yes, participants can exit the study at any time and will still receive compensation for the duration of their involvement.