


The article addresses best practices for ensuring data integrity within the pharmaceutical industry, underscoring the significance of principles such as ALCOA (Attributable, Legible, Contemporaneous, Original, Accurate) alongside the necessity for robust information management systems.
It emphasizes that adherence to these standards, combined with the utilization of advanced technologies like electronic lab notebooks and blockchain, can markedly enhance compliance and quality.
This, in turn, plays a crucial role in safeguarding patient safety and ensuring product efficacy in an environment that is becoming increasingly regulated.
The pharmaceutical industry operates under an unwavering commitment to data integrity, a cornerstone of compliance and quality assurance. As regulatory scrutiny intensifies, organizations face the pressing challenge of maintaining accurate and reliable information throughout the lifecycle of drug development. This article delves into best practices that not only uphold the core principles of data integrity but also address common pitfalls that can jeopardize patient safety and product efficacy.
How can pharmaceutical companies effectively navigate the complexities of data management while ensuring adherence to stringent standards? Exploring the intersection of technology and robust information management systems reveals critical strategies for overcoming these challenges and enhancing overall data integrity.
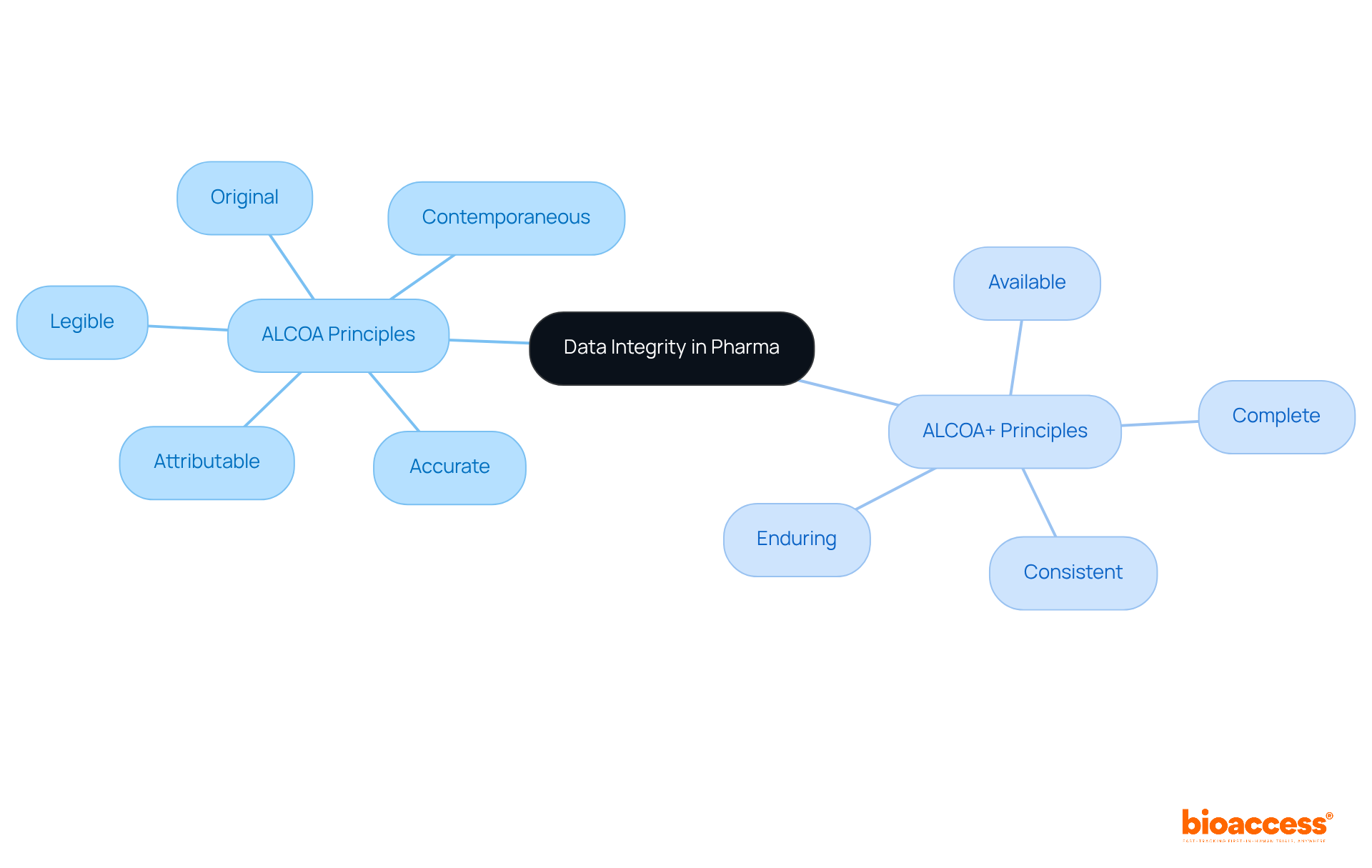
The accuracy, consistency, and reliability of information throughout its lifecycle are crucial for maintaining data integrity in pharma. The fundamental concepts of information accuracy are encapsulated by the acronym ALCOA, which stands for:
These principles are crucial for ensuring data integrity in pharma by not only gathering and documenting information correctly but also preserving it over time. Compliance with ALCOA standards is essential for meeting regulatory requirements, thereby safeguarding the safety and efficacy of pharmaceutical products. Organizations must implement robust information management systems that adhere to these principles, fostering trust and accountability in their research and development processes.
The significance of data integrity in pharma is underscored by increasing regulatory scrutiny, with agencies emphasizing the necessity for precise and dependable information in clinical trials. For instance, the application of ALCOA principles has been shown to enhance information quality and compliance, as evidenced by case studies where firms adopted advanced technologies such as AuditSafe software. This software not only timestamps interactions but also ensures that all records are assignable to the individuals responsible for their creation, thereby promoting accountability.
Moreover, the ALCOA+ framework, which builds upon the original principles by incorporating:
further strengthens the focus on information reliability. By adhering to these principles, pharmaceutical firms can enhance data integrity in pharma, mitigating risks associated with information manipulation and errors, and ultimately leading to improved patient safety and product quality. As the sector evolves, the commitment to information accuracy will remain a fundamental aspect of effective drug research and regulation.
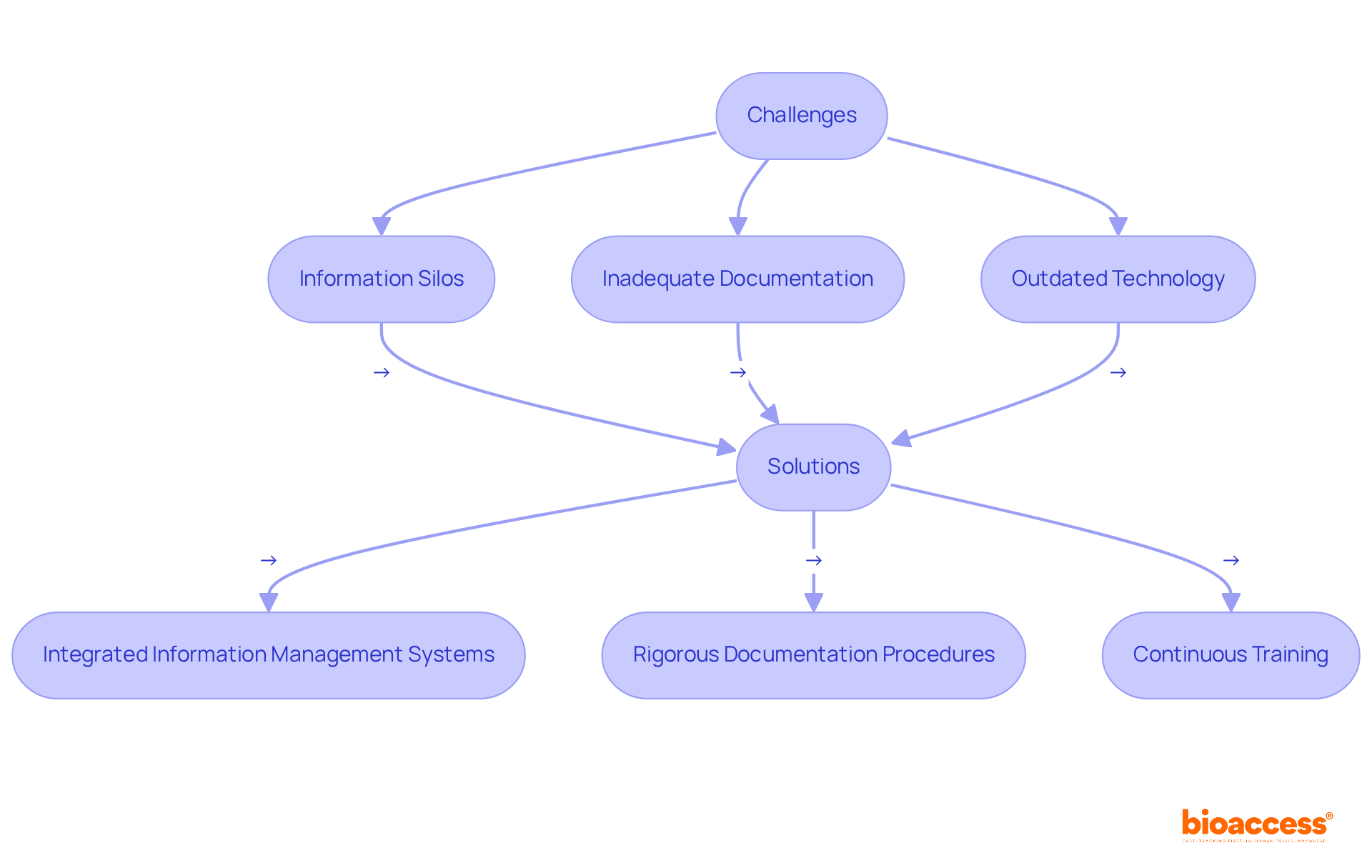
Preserving data integrity in pharma presents significant challenges, primarily due to issues such as information silos, inadequate documentation practices, and reliance on outdated technology. For instance, data silos can lead to inconsistencies and inaccuracies, as vital information remains confined within departments, obstructing collaboration and comprehensive analysis. This fragmentation may result in erroneous conclusions, wasted resources, and jeopardized patient safety. Furthermore, insufficient documentation methods often yield incomplete records, complicating the tracing of information origins and undermining compliance with regulatory standards.
To address these challenges, organizations must implement integrated information management systems that support data integrity in pharma by facilitating real-time sharing across departments, effectively dismantling silos and enhancing accuracy. Establishing rigorous documentation procedures is essential to ensure that all information is logged concurrently and remains clear and attributable. Continuous training for personnel on information reliability principles, including the importance of precise record-keeping, is vital for fostering a culture of compliance and quality. By prioritizing these solutions, pharmaceutical companies can significantly bolster data integrity in pharma, ensuring trustworthy and valid data throughout the research and development lifecycle.
Integrating the ALCOA principles—Attributable, Legible, Contemporaneous, Original, and Accurate—into information management practices can further elevate compliance and information quality. Moreover, statistics indicate that over 30% of registrants encounter significant challenges in merging and integrating information within pharmaceutical research and development, underscoring the necessity of addressing information silos. Insights from experts, such as Jennifer Shin, emphasize the importance of curiosity and diligence in information practices, reinforcing the need for meticulous record-keeping. By implementing these strategies and remaining cognizant of potential challenges, organizations can navigate the complexities of information reliability more effectively.
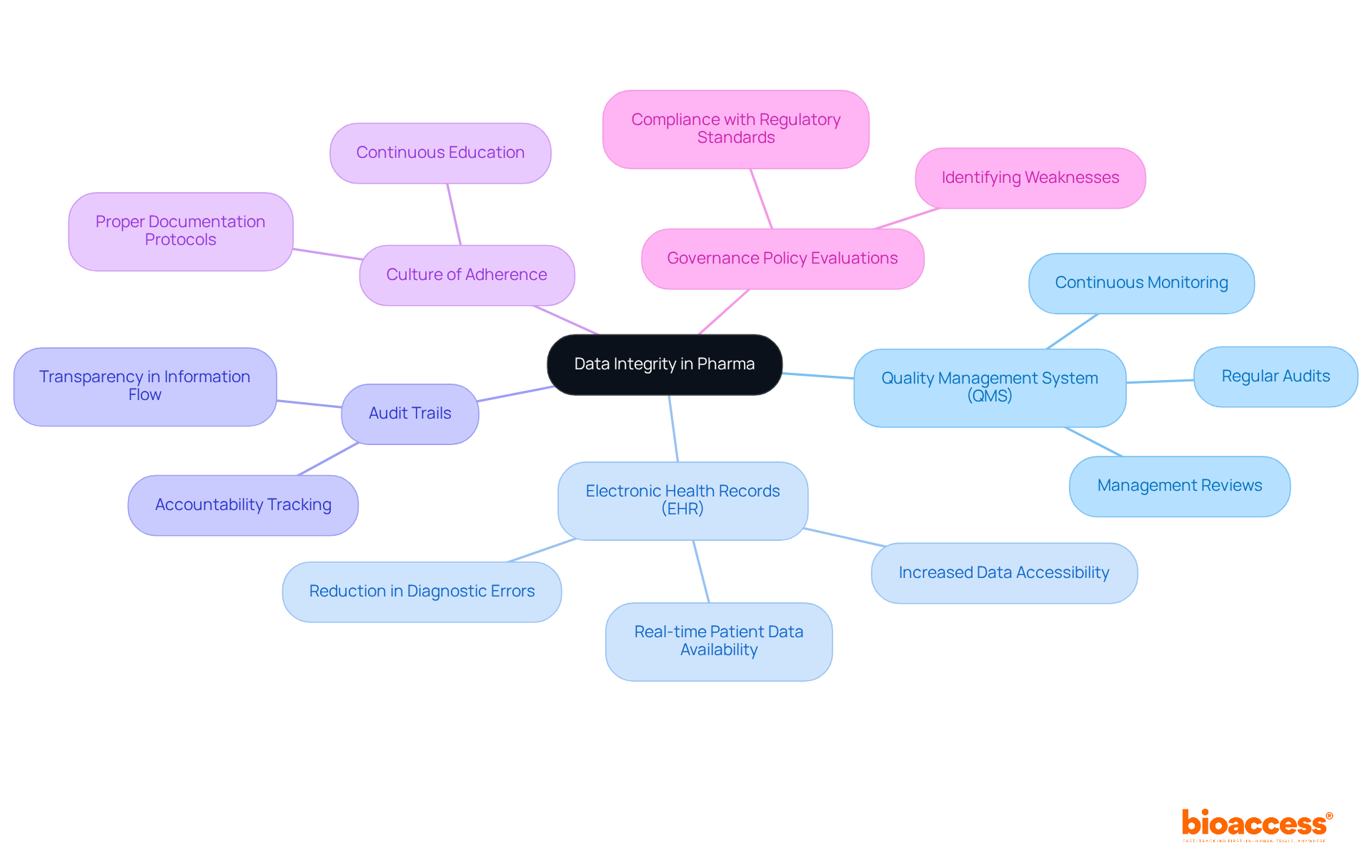
To ensure data integrity in pharma, organizations must adopt several best practices. A strong quality management system (QMS) is essential for maintaining data integrity in pharma, incorporating regular audits and evaluations of information management processes to pinpoint and correct inconsistencies.
For instance, the implementation of electronic health record (EHR) systems in U.S. hospitals surged from approximately 10% in 2008 to over 75% by 2015, with more than 95% of hospitals adopting certified EHR systems by 2021. This significant shift has greatly improved information precision and accessibility.
These systems must include audit trails to monitor changes and ensure accountability, while tracking information lineage is vital for data integrity in pharma, offering complete transparency over information flow—an essential aspect for regulatory audits and troubleshooting.
Furthermore, fostering a culture of adherence is crucial; continuous education for all staff involved in information management should emphasize the importance of data integrity in pharma and specific methods that reinforce it, such as proper documentation and information entry protocols.
Additionally, routine evaluations of governance policies are vital to identify weaknesses and ensure compliance with new regulatory standards. By integrating these strategies, organizations can adeptly navigate the complex regulatory landscape while maintaining high-quality standards in their operations.
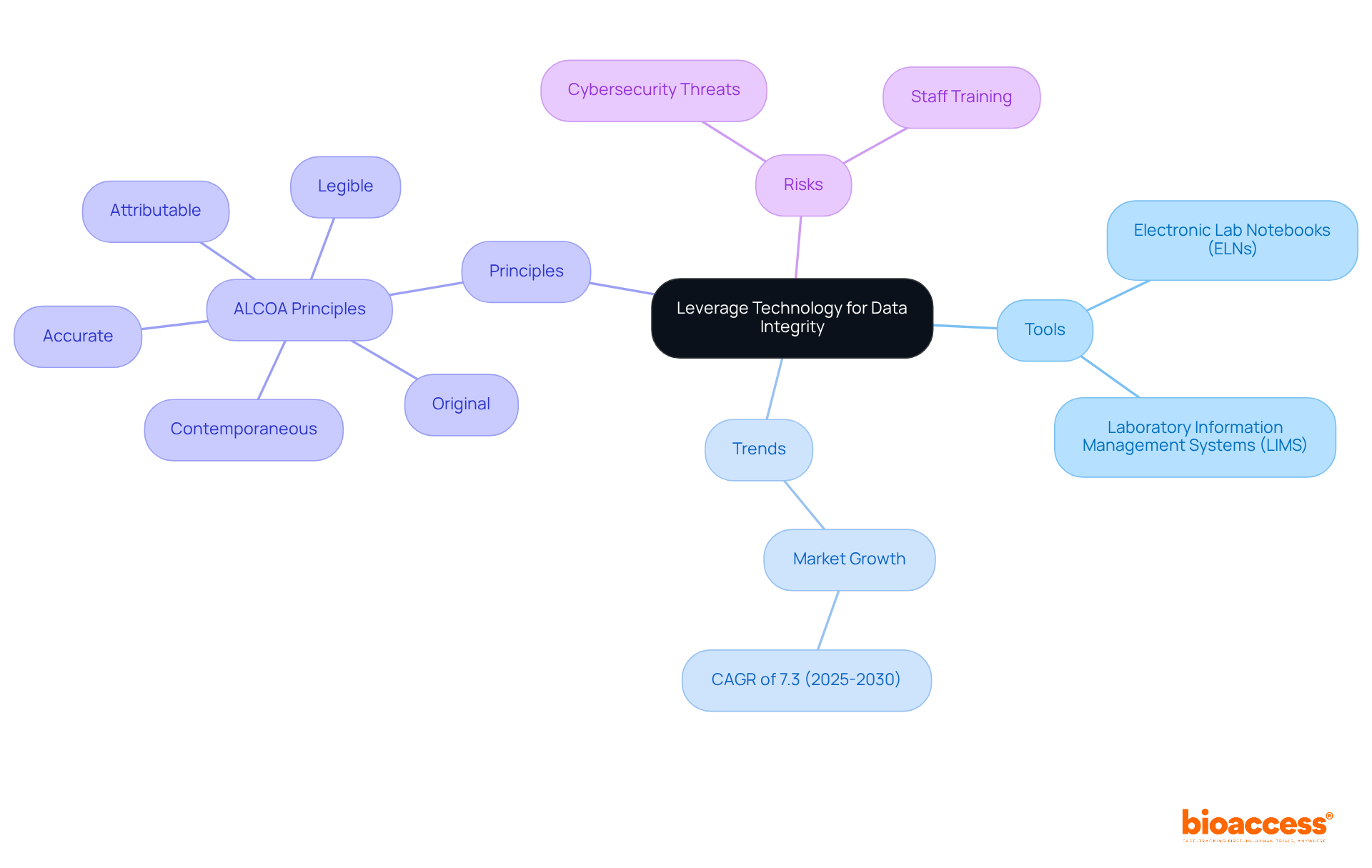
In the pursuit of information integrity, the utilization of technology is paramount. Tools such as electronic lab notebooks (ELNs) and laboratory information management systems (LIMS) significantly enhance information accuracy and traceability. Notably, the electronic lab notebook market is projected to grow at a CAGR of 7.3% from 2025 to 2030, underscoring the increasing importance of adopting these technologies within the pharmaceutical industry. These systems automate information entry and provide real-time access to details, thereby minimizing the risk of human error.
Furthermore, the application of blockchain technology offers a secure and transparent method for monitoring information changes, ensuring that all alterations are documented and verifiable. Organizations should also consider employing artificial intelligence (AI) and machine learning (ML) to proactively examine patterns and detect potential reliability issues. The ALCOA principles—Attributable, Legible, Contemporaneous, Original, and Accurate—serve as essential guidelines for preserving information reliability, with technology playing a vital role in supporting these principles.
However, it is crucial to acknowledge the risks associated with digitalization in the pharmaceutical sector, including cybersecurity threats and information breaches. Adequate training for staff managing information is essential to preserve accuracy and prevent mishandling. Moreover, regular and comprehensive assessments of information are necessary to ensure precision and adherence to regulations. As Dr. Filipa Mascarenhas-Melo notes, companies that are slow to adopt digital technologies may find themselves at a competitive disadvantage. By embracing these technological innovations, pharmaceutical companies can bolster their data integrity frameworks and ensure compliance with regulatory standards.
Ensuring data integrity in the pharmaceutical industry is vital for maintaining compliance and quality throughout the research and development lifecycle. By adhering to core principles such as ALCOA and ALCOA+, organizations can safeguard the accuracy, consistency, and reliability of their data. This commitment not only fulfills regulatory requirements but also enhances patient safety and product quality—elements that are paramount in the pharmaceutical sector.
Key challenges such as information silos, inadequate documentation, and outdated technology have been identified as significant barriers to achieving data integrity. Solutions like implementing integrated information management systems, fostering a culture of adherence, and leveraging advanced technologies such as electronic lab notebooks and blockchain emerge as effective strategies to overcome these hurdles. By prioritizing these best practices, pharmaceutical companies can significantly enhance their data management processes and ensure the integrity of their research outcomes.
Ultimately, ongoing dedication to data integrity is crucial for the pharmaceutical industry as it navigates an increasingly complex regulatory landscape. Embracing innovative technologies and implementing robust information management practices will not only enhance compliance but also foster a culture of quality assurance. Organizations are encouraged to take proactive steps in strengthening their data integrity frameworks, ensuring that they remain competitive and trustworthy in delivering safe and effective pharmaceutical products.
What is data integrity in the pharmaceutical industry?
Data integrity in pharma refers to the accuracy, consistency, and reliability of information throughout its lifecycle, which is crucial for maintaining the safety and efficacy of pharmaceutical products.
What does the acronym ALCOA stand for?
ALCOA stands for Attributable, Legible, Contemporaneous, Original, and Accurate. These principles are essential for ensuring data integrity by guiding how information is gathered, documented, and preserved.
Why is compliance with ALCOA standards important?
Compliance with ALCOA standards is important for meeting regulatory requirements, which helps safeguard the safety and efficacy of pharmaceutical products.
How does increasing regulatory scrutiny affect data integrity in pharma?
Increasing regulatory scrutiny emphasizes the necessity for precise and dependable information in clinical trials, highlighting the importance of data integrity in the pharmaceutical industry.
What role does technology play in enhancing data integrity?
Advanced technologies, such as AuditSafe software, enhance data integrity by timestamping interactions and ensuring all records are assignable to responsible individuals, thereby promoting accountability and compliance.
What additional principles are included in the ALCOA+ framework?
The ALCOA+ framework includes additional principles: Complete, Consistent, Enduring, and Available, which further strengthen the focus on information reliability.
How do the principles of ALCOA and ALCOA+ contribute to patient safety?
By adhering to the principles of ALCOA and ALCOA+, pharmaceutical firms can enhance data integrity, mitigate risks associated with information manipulation and errors, and ultimately lead to improved patient safety and product quality.