


The role of a clinical study monitor is critical in overseeing clinical research, ensuring compliance with regulatory standards, and safeguarding participant safety throughout the study process. Effective monitoring not only enhances data integrity and participant trust but also accelerates the development of new treatments. This underscores the monitor's importance in achieving successful research outcomes, making their contributions indispensable in the clinical research landscape.
The intricate world of clinical trials is built upon a myriad of roles, yet none are as pivotal as that of the clinical study monitor. As guardians of compliance and data integrity, these professionals ensure that research is conducted ethically and effectively, safeguarding participant welfare and the validity of findings.
With the landscape of medical research continually evolving, the key skills and responsibilities that define the success of a clinical study monitor become increasingly critical.
How do these experts navigate the complexities of modern clinical trials? This exploration delves into the vital contributions of clinical study monitors, revealing their indispensable role in shaping the future of medical research.
A clinical study monitor, also referred to as a Research Associate (CRA), plays a pivotal role in overseeing research studies, ensuring adherence to regulatory standards and study protocols. Their core responsibilities include:
All while ensuring that data is collected accurately and ethically. Acting as a crucial link between the sponsor and the study site, CRAs facilitate communication and promptly address any issues that may arise during the research.
Regular site visits form a fundamental part of a CRA's responsibilities, enabling them to confirm that the study is conducted according to the established protocol and that participant safety remains paramount. This hands-on approach is essential, as effective monitoring by a clinical study monitor not only ensures compliance with Good Clinical Practice (GCP) guidelines but also enhances the overall integrity of the study. Indeed, statistics reveal that 91% of research participants rated their experience as 'Excellent' or 'Good,' underscoring the critical role CRAs play in fostering a positive environment for participants.
Moreover, the clinical study monitor is responsible for reviewing reporting documentation, ensuring compliance with protocols and Standard Operating Procedures (SOPs), while addressing ethical and regulatory considerations. Their expertise is indispensable in maintaining the safety profile and efficacy of the therapeutic under study, as they gather and report vital medical information, including adverse effects and treatment considerations. With bioaccess® facilitating regulatory approvals in 6-8 weeks, compared to the typical 6-12 months in the US/EU, the role of CRAs continues to evolve, enhancing their ability to manage studies efficiently and ensure participant welfare across diverse research sites.
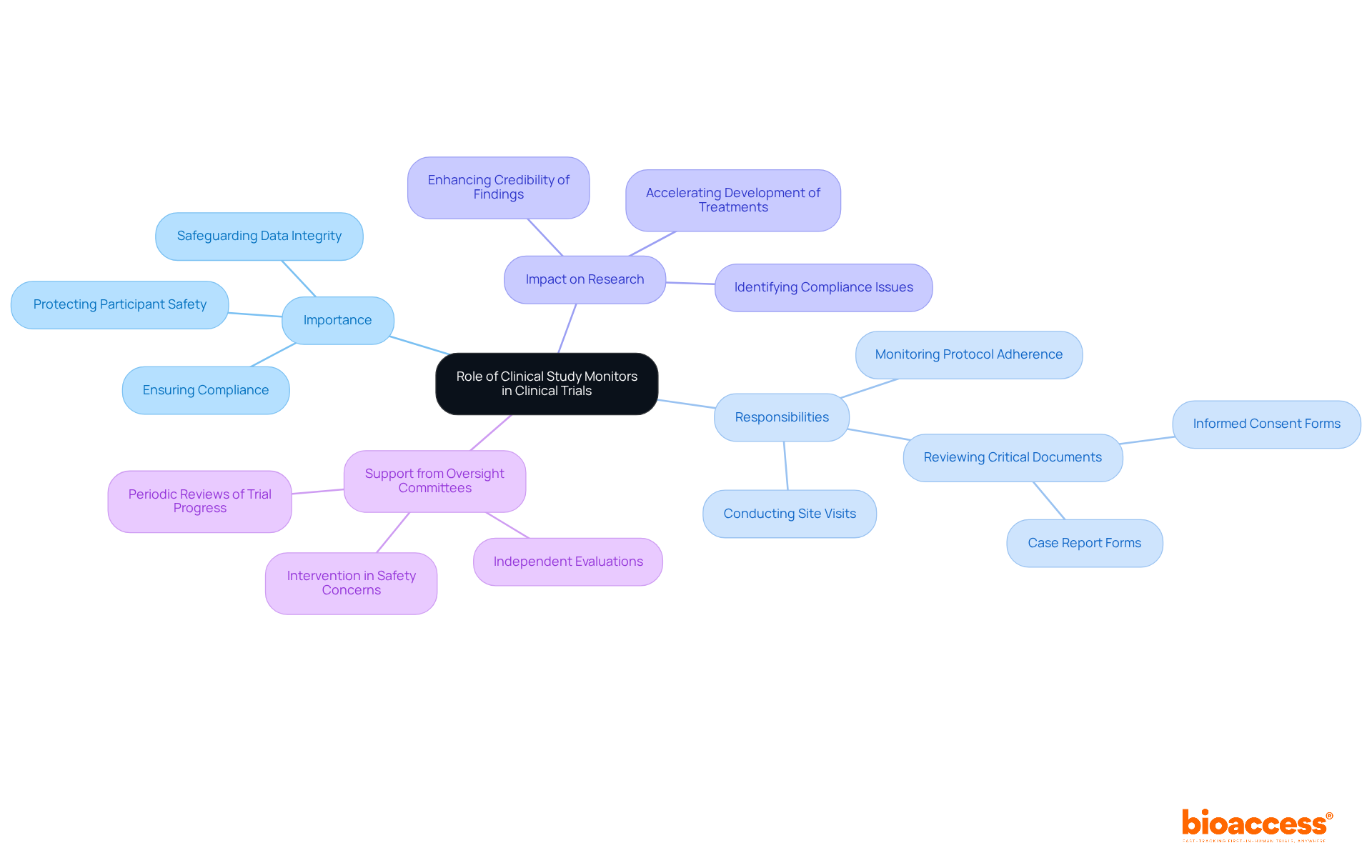
Clinical study monitors are essential to the research process, ensuring adherence to Good Practice (GCP) guidelines and regulatory standards. The clinical study monitor plays a pivotal role in safeguarding data integrity, which directly influences the validity of experimental outcomes. Research indicates that studies with dedicated clinical study monitors achieve compliance rates significantly higher than those without, underscoring their importance in protecting participant safety and mitigating risks associated with medical research.
For example, effective monitoring practices have been shown to enhance the credibility of findings by swiftly identifying and resolving compliance issues. Furthermore, their expertise in navigating complex regulatory landscapes facilitates a smoother progression of studies, ultimately accelerating the development of new treatments and medical devices.
By conducting regular site visits, reviewing critical documents such as informed consent forms and case report forms, and ensuring adherence to protocols, the clinical study monitor plays an integral role in maintaining the integrity of research, thereby fostering trust in research outcomes.
Additionally, oversight committees bolster the efforts of monitor personnel by providing independent evaluations of research progress and participant safety, further elevating the overall quality of medical research.
The comprehensive medical research management services offered by bioaccess, which include feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting on adverse events, emphasize the indispensable role that a clinical study monitor plays in achieving successful research outcomes.
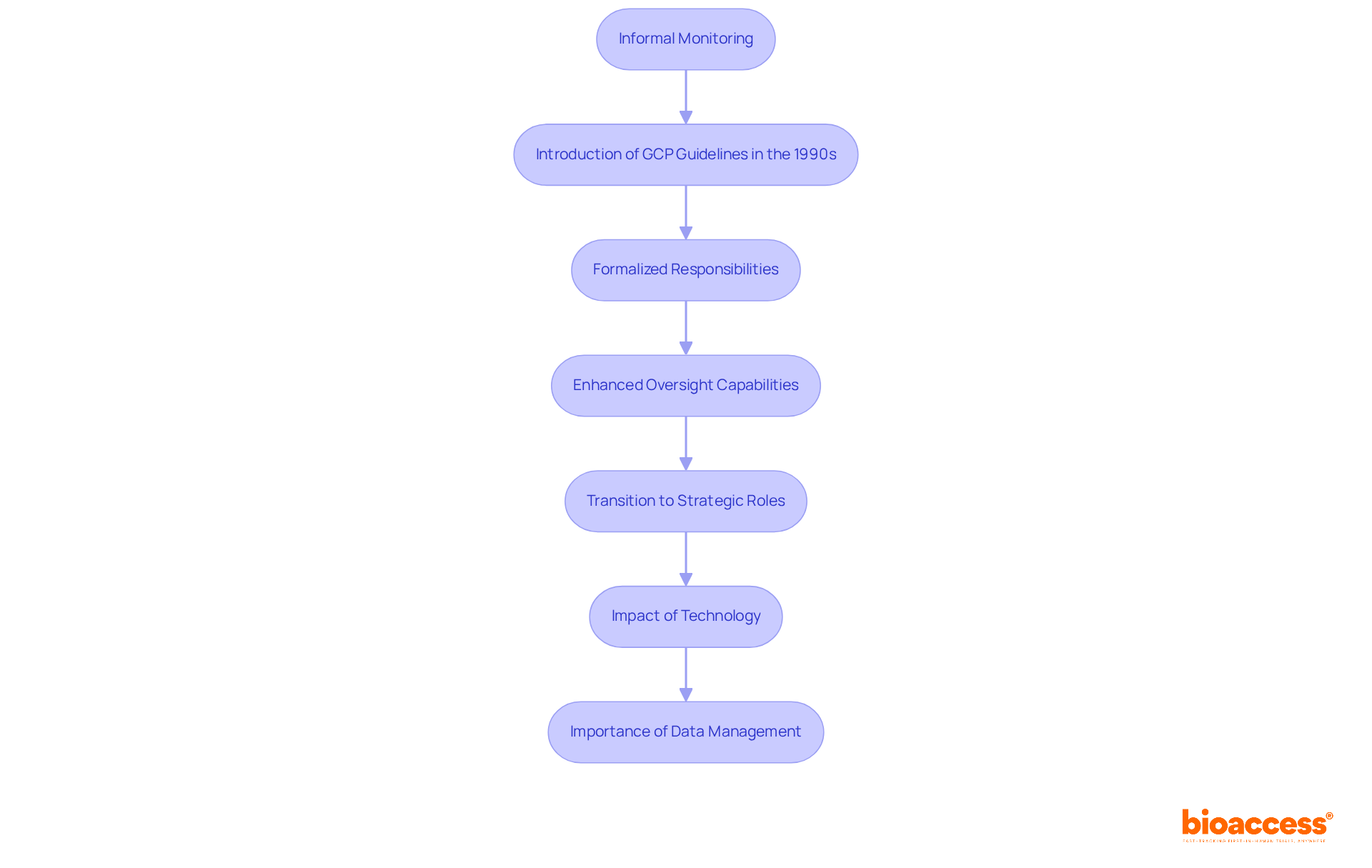
The role of the clinical study monitor has undergone a significant transformation over the past few decades. Initially, monitoring was an informal process, often managed by sponsors or investigators themselves. However, as medical studies grew in complexity and regulatory scrutiny intensified, the necessity for a clinical study monitor became evident. The introduction of Good Practice (GCP) guidelines in the 1990s marked a pivotal moment, formalizing the responsibilities of monitors and underscoring the need for independent oversight. This shift not only enhanced the integrity of medical studies but also established a framework for accountability and compliance.
Today, the clinical study monitor is essential to the research process, equipped with advanced tools and technologies that significantly enhance their oversight capabilities. The evolution of the Clinical Research Associate (CRA) role has mirrored this development, with CRAs transitioning from administrative tasks to strategic positions focused on data analysis and compliance. Statistics indicate substantial growth in the CRA profession, reflecting the increasing demand for skilled professionals in this domain. As the research studies market is projected to expand at an annual compound rate of 5.8% until 2030, the role of a clinical study monitor becomes increasingly important.
At bioaccess®, our research team, with over 20 years of experience in Medtech, is committed to advancing medical devices through Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). Our comprehensive research study management services encompass:
This dedication not only facilitates successful acquisitions in Medtech through ethical medical data and strategic planning but also contributes to local economies by creating jobs, fostering economic growth, and enhancing healthcare outcomes.
Industry leaders emphasize this evolution: "Data is indeed everywhere, and those who know how to handle it are in a very powerful position," highlighting the critical role of data management in monitoring. Furthermore, the continuous advancement in technology and techniques is reshaping the landscape of research oversight, ensuring that the role of the clinical study monitor remains at the forefront of maintaining integrity and patient safety. Additionally, the rise of virtual trials necessitates that Research Monitors adapt to new methodologies, further illustrating the dynamic nature of their role.
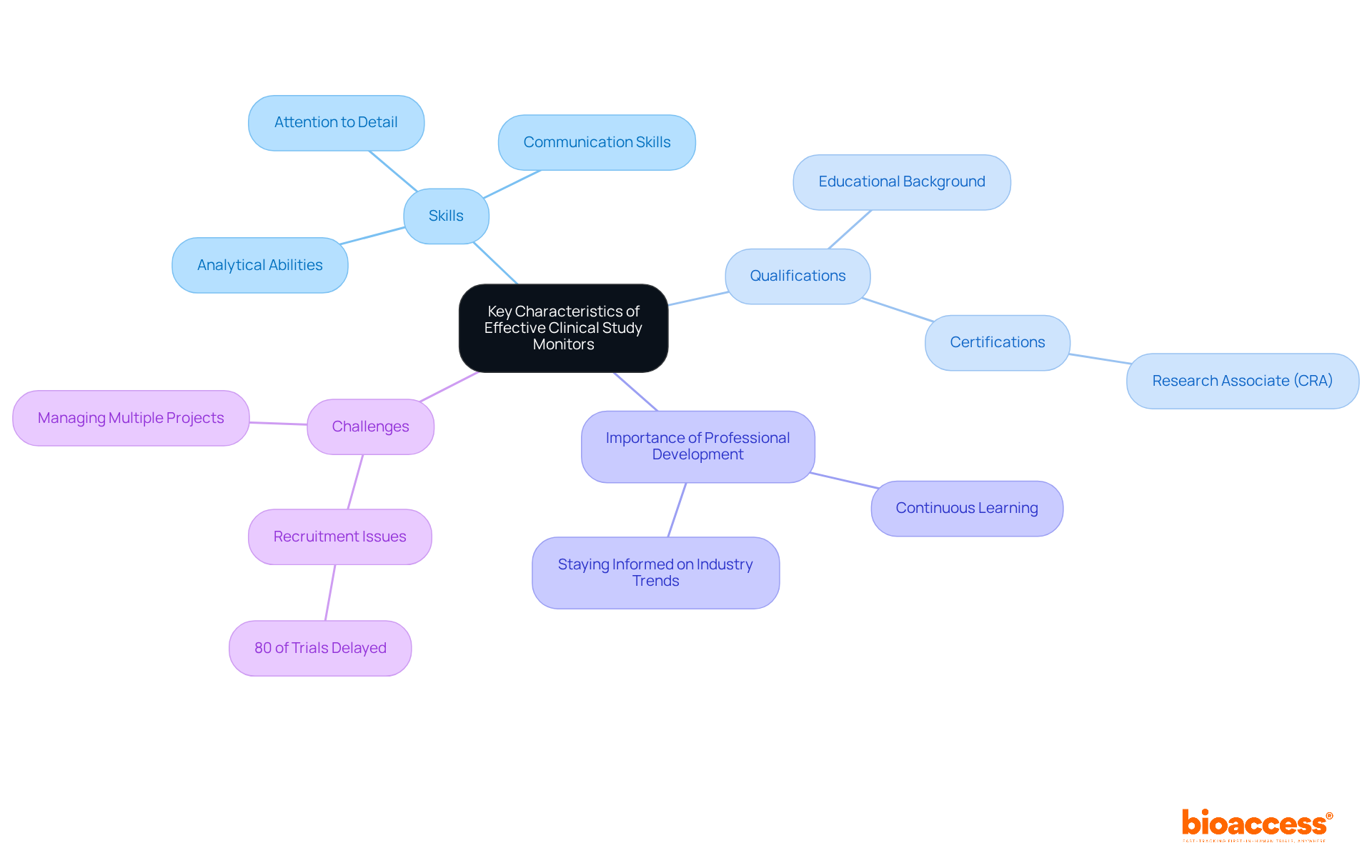
Effective Study Monitors represent a unique blend of skills and qualifications that enable them to excel in their roles. Key characteristics include:
A thorough understanding of research regulations and Good Clinical Practice (GCP) is essential, coupled with the capability to manage multiple projects simultaneously. Typically, these professionals hold degrees in life sciences or related fields, with many earning certifications such as the Research Associate (CRA) credential. Continuous professional development is vital, as it ensures monitors stay informed about industry trends and regulatory changes, which is crucial for maintaining compliance and guaranteeing the success of research studies.
Notably, data indicates that over 80% of research studies face delays due to recruitment challenges, underscoring the importance of effective communication strategies employed by Research Monitors to engage and retain participants. Furthermore, the educational backgrounds of Research Associates often encompass a diverse range of disciplines, enhancing a holistic approach to trial management.
Ultimately, the success of a clinical study monitor depends on their ability to integrate these competencies with a proactive mindset, fostering an environment that promotes innovation and efficiency in clinical research.
The role of a clinical study monitor is crucial in ensuring the success and integrity of clinical trials. By overseeing research studies and ensuring compliance with regulatory standards, these professionals serve as the essential bridge between sponsors and study sites. Their responsibilities encompass:
All while prioritizing participant safety and data accuracy.
This article has shared critical insights regarding the importance of clinical study monitors in maintaining the integrity of research. Their expertise not only safeguards participant welfare but also enhances the credibility of study findings. Notably, studies employing dedicated monitors achieve significantly higher compliance rates, directly impacting the quality of medical research outcomes. Furthermore, the evolution of this role reflects an increasing demand for skilled professionals adept at navigating complex regulatory landscapes and employing advanced monitoring techniques.
In light of these insights, it is evident that the role of clinical study monitors will continue to evolve, particularly as the landscape of clinical trials changes with technological advancements and new methodologies. The future of clinical research relies heavily on these professionals, who not only ensure compliance and safety but also foster an environment conducive to innovation and efficiency. As the industry progresses, the commitment to developing skilled clinical study monitors will be crucial in advancing medical research and improving healthcare outcomes.
What is the role of a Clinical Study Monitor?
A Clinical Study Monitor, also known as a Research Associate (CRA), oversees research studies to ensure adherence to regulatory standards and study protocols while ensuring that data is collected accurately and ethically.
What are the core responsibilities of a Clinical Study Monitor?
The core responsibilities include site selection, initiation, monitoring, and closeout of clinical studies.
How does a Clinical Study Monitor ensure participant safety?
They conduct regular site visits to confirm that the study is conducted according to the established protocol and prioritize participant safety throughout the research process.
What guidelines do Clinical Study Monitors follow?
They ensure compliance with Good Clinical Practice (GCP) guidelines, which enhance the overall integrity of the study.
What is the significance of the statistics related to participant experience?
Statistics show that 91% of research participants rated their experience as 'Excellent' or 'Good,' highlighting the critical role CRAs play in fostering a positive environment for participants.
What documentation does a Clinical Study Monitor review?
They review reporting documentation to ensure compliance with protocols and Standard Operating Procedures (SOPs), addressing ethical and regulatory considerations.
How do Clinical Study Monitors contribute to the safety and efficacy of therapeutic studies?
They gather and report vital medical information, including adverse effects and treatment considerations, which is essential for maintaining the safety profile and efficacy of the therapeutic under study.
How has the role of Clinical Study Monitors evolved?
With advancements like bioaccess® facilitating regulatory approvals in 6-8 weeks, the role of CRAs has evolved to enhance their ability to manage studies efficiently and ensure participant welfare across diverse research sites.