


Colombia has enacted a groundbreaking new law that fundamentally overhauls its clinical trial landscape, establishing what is arguably the most advanced and attractive research ecosystem in Latin America. This legislation is not an incremental update; it is a paradigm shift designed to solve historical bottlenecks and position the country as a global leader in medical innovation.
For decades, Colombia's research potential was constrained by an outdated regulatory framework, primarily Resolutions 8430 of 1993 and 2378 of 2008, which created administrative hurdles, inefficient approval processes, and requirements misaligned with international standards.1 The new law directly confronts these challenges by holistically integrating unprecedented regulatory speed, legally mandated international quality standards, world-class financial incentives, and end-to-end digitalization. Its explicit goal is to foster competitiveness, guarantee the highest ethical standards, and establish Colombia as a global hub for research, with a strategic focus on advanced fields like radiopharmaceuticals and theranostics.1
The law’s most disruptive feature is its risk-based approval model, which delivers legally guaranteed speed and predictability, addressing what has been a major deterrent for sponsors in the region.2
The legislation introduces the principle of Confianza Supervisada, or "Supervised Trust".1 This represents a philosophical shift away from a slow, sequential, and bureaucratic review process. Instead, it places trust in the rigorous ethical and scientific evaluation performed by internationally accredited Ethics Committees (Comités de Ética en Investigación - CEI), a move designed to eliminate the redundant evaluations that previously caused significant delays.1
Under this model, the regulatory authority, the National Food and Drug Surveillance Institute (INVIMA), shifts its primary focus. Rather than re-evaluating every aspect of every study, INVIMA concentrates its expert resources on high-risk investigations and robust post-approval surveillance, including inspections and vigilance.1 This distribution of responsibility empowers accredited CEIs as the frontline evaluators, streamlining the entire process.
The law codifies specific, aggressive timelines based on a study's risk profile, leveraging a mechanism known as "tacit approval" (silencio administrativo positivo) to ensure decisions are made swiftly.1
When benchmarked against other key markets, Colombia's new timelines provide a clear and compelling competitive advantage.
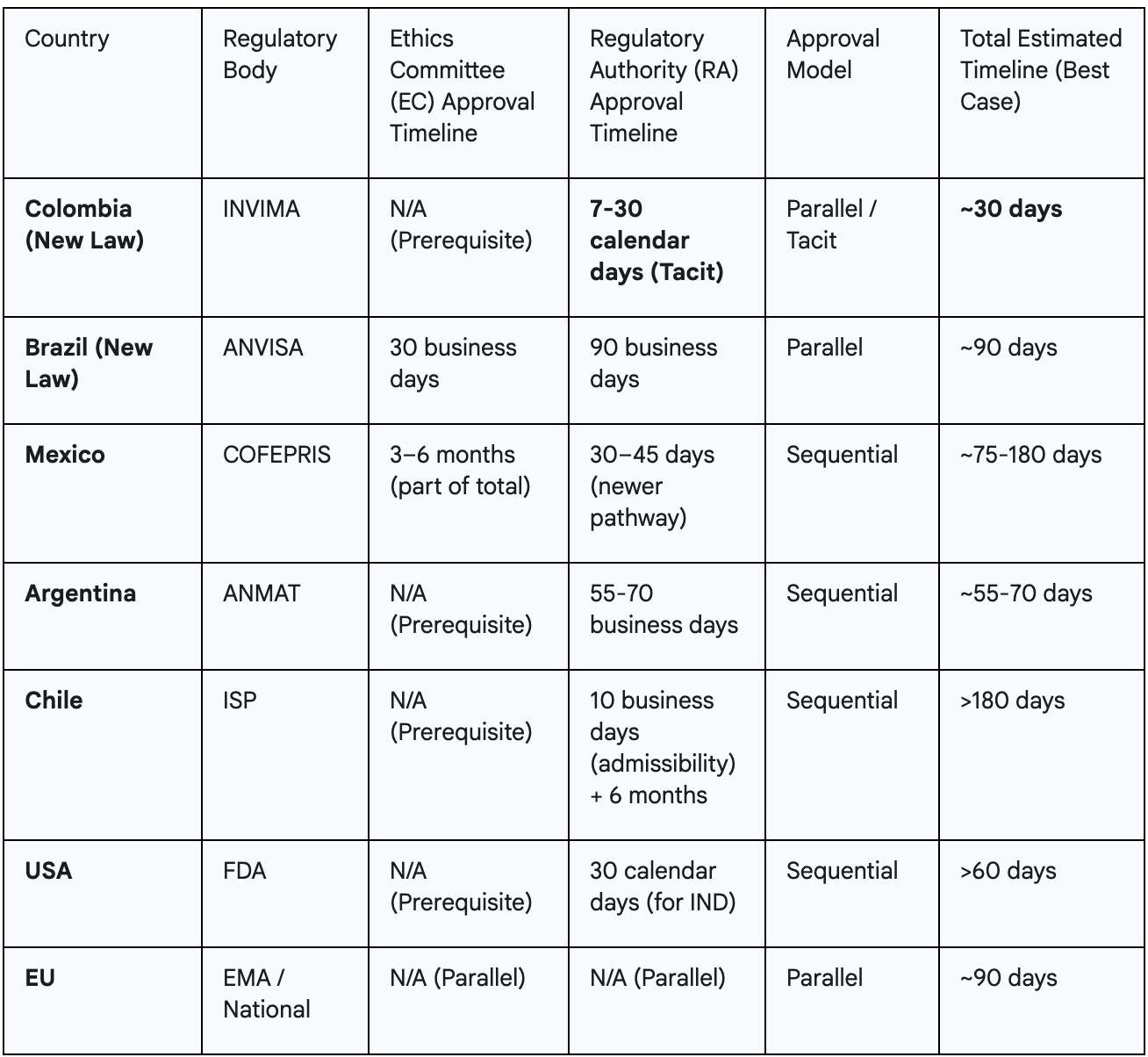
The law's greatest innovation is not just speed, but legally guaranteed predictability. Historically, sponsors operating in Latin America have faced highly variable and uncertain approval timelines, with research showing the region has some of the longest cycle times from site selection to first-patient-in.11 This uncertainty complicates global trial coordination, budget forecasting, and resource allocation.
The Colombian law replaces this ambiguity with a fixed, legally mandated deadline. The "tacit approval" mechanism fundamentally shifts the burden of action onto the regulator; the default outcome after the 30-day period is approval, not continued silence or further waiting.1 For a sponsor, this transforms the approval timeline from an unpredictable risk factor into a reliable planning parameter. This legal certainty allows sponsors to set firm study start dates, coordinate logistics with confidence, and drastically reduce the financial and operational costs associated with waiting, thereby de-risking the most volatile phase of study startup.
The new law embeds globally recognized quality standards directly into the national legal framework, creating an unparalleled guarantee of operational excellence and data integrity that will be accepted by regulators worldwide.
The legislation moves away from a localized, and often outdated, national certification system. Instead, it mandates that key players in the ecosystem be credentialed by globally respected bodies.1
To remove any ambiguity, the law formally adopts the latest versions of international "gold standards" as legally binding. This includes the International Council for Harmonisation's Good Clinical Practice guidelines (ICH-E6) for all trials and the ISO 14155 standard for trials involving medical devices.1
This legislative approach represents a uniquely intelligent model of quality assurance. The previous system, which relied on a national certification standard, was criticized for becoming obsolete and misaligned with global practices.1 Instead of simply updating this national standard, the new law eliminates it entirely in favor of legally recognizing established international accreditations.1
This strategy has a powerful dual effect. First, it provides instant credibility. A sponsor can be confident that an AAHRPP-accredited center in Bogotá meets the exact same rigorous standards as a top-tier institution in the United States or Europe, effectively eliminating country-specific risk related to data quality and site performance. Second, it future-proofs the regulatory system. As global standards evolve (e.g., the implementation of ICH E6(R3)), Colombia's legal requirements automatically update in tandem, without the need for slow legislative reform. This dynamic peg to the global gold standard builds unparalleled confidence for sponsors, assuring them that data generated in Colombia will be readily accepted by the FDA, EMA, and other major regulatory bodies.
The law establishes a powerful and sophisticated financial framework designed to make Colombia a highly competitive destination for both established pharmaceutical companies and cash-sensitive biotech startups.
The law mandates the creation of a comprehensive tax incentive regime for investments in clinical research, which may include special deductions, tax credits, and exemptions from tariffs on imported equipment and supplies.1
A key provision is the explicit consideration of a refundable tax credit for investments in research, development, and innovation (R&D&i).1 This is a particularly modern and attractive mechanism for early-stage companies with limited or no revenue. The law also calls for preferential incentives for high-priority areas, such as research into radiopharmaceuticals and rare diseases.1
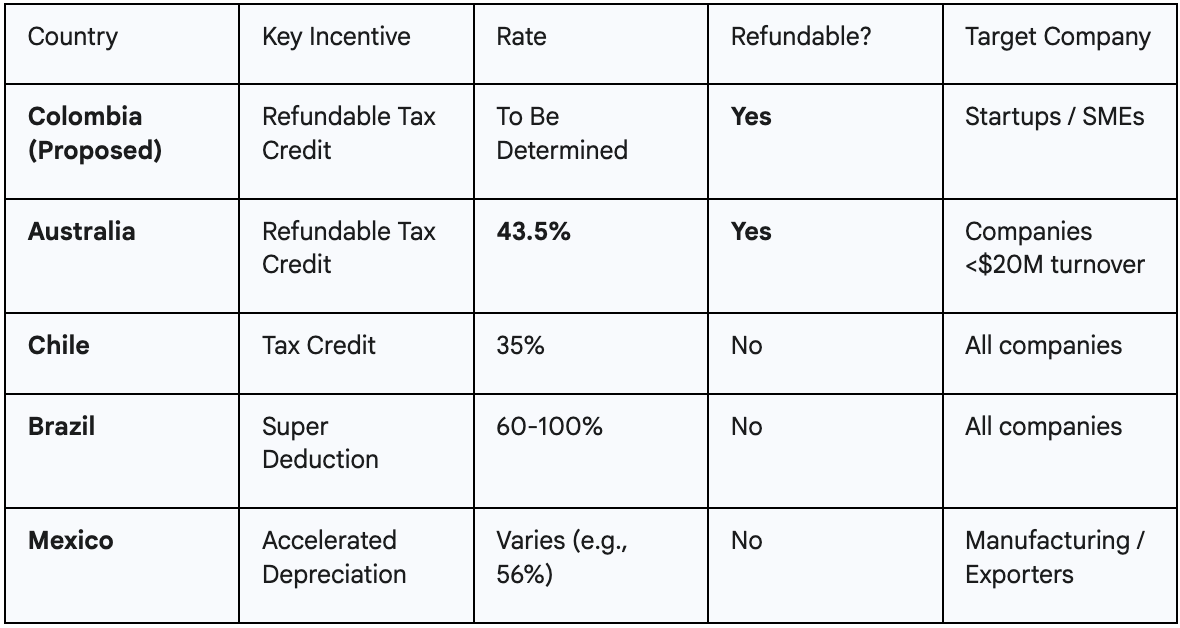
Colombia's proposed incentives are designed not just to exist, but to compete directly with the most attractive R&D schemes in the world, most notably Australia's highly successful model.
The law creates the Fondo para el Fortalecimiento de la Investigación Clínica (FORCLINC), a strategic public-private fund designed to ensure the long-term health of the research ecosystem.1
The law's financial framework is strategically designed to attract two distinct types of sponsors. Large, profitable pharmaceutical companies are primarily motivated by traditional tax deductions and credits, which reduce their overall tax burden on existing profits.21 The new law provides for these mechanisms.1
However, pre-revenue biotech startups have no tax liability to reduce, making such deductions worthless. Their primary constraint is cash flow. For these companies, the proposed refundable tax credit is a game-changer.1 Modeled after Australia's successful program, this incentive acts as a direct cash rebate from the government—a non-dilutive source of funding that can be critical for survival and growth.16
By including provisions for both types of incentives, Colombia is creating a sophisticated financial environment that is simultaneously attractive to a multinational giant seeking to lower its tax bill and a small startup that needs cash to fund its next experiment. This dual appeal provides a significant competitive advantage over countries that offer a single, one-size-fits-all incentive.
The law mandates a digital-first approach to eliminate the logistical and administrative bottlenecks that have historically plagued clinical trials in the region.
A unified digital platform, the Plataforma Digital Integrada, will be implemented for all submissions, communications, and notifications between sponsors, CEIs, and INVIMA.1 This single portal is designed to handle large volumes of data securely, facilitate coordinated and simultaneous reviews, and replace the inefficient paper-based, sequential processes of the past.1
A critical feature of the new system is the deep integration of the digital platform with the Ventanilla Única de Comercio Exterior (VUCE), Colombia's single window for foreign trade.1
The law mandates the creation of a free, public National Registry of Clinical Studies, where all approved trials must be registered before enrolling the first participant.1 In line with WHO standards, this registry will require the publication of a summary of results—whether positive, negative, or inconclusive—upon study completion, fostering a culture of transparency and contributing to the global body of scientific knowledge.
The true speed of this new system lies not just in the formal regulatory review but in its attack on the "hidden" delays that inflate timelines and budgets in emerging markets. The official approval timeline is only one part of the study startup process. Sponsors often face unpredictable delays of weeks or even months navigating customs clearance and securing import permits.1
By directly linking trial approval to a five-day, automated import authorization via the VUCE integration, the law effectively eliminates this major logistical bottleneck.1 Similarly, by pre-approving the export of biological samples, it removes another common source of delay for global studies.1 This holistic approach to time-saving radically compresses the entire end-to-end startup timeline, from initial submission to having the investigational product on-site and ready for the first patient.
Beyond general improvements, the law reveals a highly specific and ambitious national strategy: to become a dominant global hub for research in radiopharmaceuticals and theranostics.
The focus on radiopharmaceuticals and theranostics is not an afterthought; it is a central theme woven throughout the legislation. It appears in the law's core objectives, key definitions, quality requirements, and financial incentive structures.1 This signals a calculated national strategy to build a world-leading ecosystem in a specific, high-growth area of precision medicine.1
The law includes numerous provisions specifically designed to attract and support research in this advanced field:
Colombia's approach is a classic example of a "niche domination" strategy. Rather than competing broadly against established R&D giants like the US and EU across all therapeutic areas, the country is concentrating its resources to build an unparalleled ecosystem in one specific, forward-looking field. Radiopharmaceuticals and theranostics represent a newer, highly specialized, and capital-intensive area where fewer countries have deep, established infrastructure.
By creating a law that explicitly caters to every need of a theranostics sponsor—from fast-tracked cyclotron imports to specialized talent and preferential tax treatment—Colombia is sending a clear and powerful signal to the global market: if you are developing a next-generation radiopharmaceutical, this is the single best place in the world to do it. This targeted approach is far more likely to yield a dominant market position than a more generalized one.
The law’s focus on efficiency and competitiveness does not come at the expense of ethics. Instead, it strengthens patient protections, which in turn creates a more stable and successful research environment for sponsors.
The patient perspective is integrated into the governance of the entire system. The law mandates the inclusion of patient representatives and laypersons (members of the public) on all accredited Ethics Committees.1 Furthermore, patient organizations will have a formal seat on the new High-Level National Advisory Committee for Clinical Research, ensuring their voice influences national policy.1
Robust, legally enshrined patient protections are not just an ethical imperative; they are a direct driver of operational success for sponsors. Patient recruitment and retention are consistently cited as major challenges in clinical trials, with high dropout rates threatening data integrity and extending timelines.11 A key factor influencing a patient's decision to enroll and remain in a trial is trust in the system and in the investigators.25
By legally guaranteeing rights like post-trial access and providing a clear, regulated pathway for compassionate use, the law builds systemic trust. Patients and physicians are more likely to participate in a system where they know their rights are protected by law, not just by a sponsor's internal policy. This can lead to faster recruitment, lower dropout rates, and a healthier relationship between the research community and the public—ultimately benefiting the sponsor's timeline and budget.
Colombia's new clinical trial law is not a single reform but a fully integrated, strategically designed ecosystem. It creates a virtuous cycle where speed, quality, and investment reinforce one another.
The combination of unmatched regulatory speed through legally guaranteed timelines, embedded data quality via mandatory international accreditations, aggressive and sophisticated financial incentives like refundable tax credits, seamless digital operations that eliminate logistical delays, and a clear strategic vision to lead in advanced therapies creates a value proposition that is unparalleled in Latin America and highly competitive on a global scale.
For any international sponsor looking to de-risk their R&D portfolio, accelerate development timelines, ensure the highest standards of data quality, and maximize their return on investment, Colombia is no longer just another option—it is the new strategic imperative.
Q1: What are the key transition periods for the new accreditation requirements?
A: Research centers have 24 months from the law's enactment to obtain international certification. Principal investigators and key staff have 12 months to obtain their international accreditation. CROs have 12 months to complete their registration with INVIMA.1
Q2: How does the "tacit approval" process work in practice for high-risk trials?
A: After a sponsor receives approval from an accredited Ethics Committee (CEI), they submit the full dossier to INVIMA via the new digital platform. INVIMA then has a 30-calendar-day window to review the submission and issue a motivated objection. If no objection is issued by the end of day 30, the trial is legally considered approved and can begin immediately.1
Q3: What is the first step for a sponsor to submit a new trial under this law?
A: The first step is to select one of Colombia's internationally accredited Ethics Committees (CEI) and prepare the submission dossier. The application to the CEI and the formal notification to INVIMA will occur simultaneously through the new Plataforma Digital Integrada.1
Q4: Are documents in English accepted for submission?
A: Yes. The law explicitly states that key technical documents, including the clinical trial protocol, investigator's brochure, and preclinical/clinical data reports, can be submitted to both the CEI and INVIMA in English. This eliminates a significant administrative and cost barrier.1
Q5: How does the law support academic or non-commercial research?
A: The law contains a dedicated title (Title VI) for non-commercial research. It establishes conditions to facilitate these studies, such as the potential for reduced or waived regulatory fees and access to technical assistance from authorities. Furthermore, the new FORCLINC fund is specifically tasked with providing financial support to high-priority academic and public interest studies.1
1 Proyecto de Ley "Por medio de la cual se establece el marco regulatorio integral para la investigación clínica con seres humanos en Colombia..."
1 Summary of "Proyecto de Ley" focusing on key provisions and 'Exposición de Motivos'.
3 ProductLife Group. "Brazil: Shorter Timelines for CTA (Clinical Trial Applications) Assessment."
4 PSI CRO. "What to Know About Brazil's New Clinical Research Law."
5 OMC Medical. "Chile Drug Product Registration."
2 ASCO Publications. "Clinical research in Latin America: Disparities and opportunities."
24 ICON plc. "ICON in Latin America."
26 Government of Mexico. "New Cofepris Digital Platform for Research and Clinical Trials (Digipris) sets the pace in regulation."
6 Credevo. "Clinical Trial Regulatory Process in Mexico."
7 Medpace. "Argentina Overhauls Drug Approval Process..."
12 EY. "Mexico offers tax incentives to taxpayers who perform productive economic activities..."
13 Baker McKenzie. "Mexico: Federal government issues tax incentives for nearshoring..."
14 Holland & Knight. "Nearshoring: US-Mexico Announce New Tax Incentives."
25 Argentina Ministry of Health. "Argentina's Plan Nacer..."
8 FDA. "Step 3: Clinical Research."
9 Advarra. "Understanding the EU Clinical Trials Regulations Updates."
15 Australian Government. "Overview of the R&D Tax Incentive."
16 SVB. "The Advantage of Oz: Offshore Tax Rebates for LSHC R&D in Australia."
17 Sofpromed. "How can US biotech benefit from Australia tax incentive for clinical trials."
22 UT Southwestern Medical Center. "Expanded Access."
23 European Medicines Agency. "Compassionate use."
21 OECD. "R&D tax incentives."
18 OECD. "STIP Compass: R&D tax allowance - Brazil."
10 Clinical Leader. "New Law Expected To Boost Clinical Research In Brazil."
26 Government of Mexico. "New Cofepris Digital Platform for Research and Clinical Trials (Digipris) sets the pace in regulation."
6 Credevo. "Clinical Trial Regulatory Process in Mexico."
19 WIPO. "Chile reforms R&D law."
20 OECD. "STIP Compass: R&D tax credit for intramural and extramural expenses - Chile."
11 Applied Clinical Trials. "New Benchmarks for Trial Initiation Activities."