


Successful corrective and preventive actions (CAPA) in clinical research hinge on several key steps:
These structured steps not only resolve existing problems but also cultivate a culture of continuous improvement and compliance within clinical research organizations. This, in turn, enhances the quality of medical studies, reinforcing the critical role of CAPA in advancing clinical research excellence.
Corrective and preventive actions (CAPA) serve as the backbone of quality assurance in clinical research, ensuring that studies uphold integrity and compliance amidst the complexities of regulatory standards. This article explores the essential steps for effectively implementing CAPA, underscoring the benefits of a structured approach that not only addresses existing issues but also anticipates future challenges. As organizations pursue excellence, the pivotal question arises: how can they seamlessly integrate CAPA with their quality management systems to cultivate a culture of continuous improvement and guarantee patient safety?
Corrective and preventive actions (CAPA) are indispensable in medical studies, as they serve as systematic procedures designed to identify, examine, and rectify issues that may compromise the quality and integrity of study results. Corrective actions are aimed at resolving existing problems, while preventive actions focus on thwarting potential issues before they manifest. In the realm of clinical trials, particularly in 2025, the significance of corrective and preventive actions (CAPA) cannot be overstated; they are critical for ensuring compliance with regulatory standards, enhancing patient safety, and upholding the credibility of research outcomes.
bioaccess® provides a comprehensive suite of services in Colombia, including:
These services are all essential for the implementation of corrective and preventive actions (CAPA). By leveraging our ability to secure ethical approvals within 4-6 weeks and achieve enrollment rates that are 50% faster than conventional markets, bioaccess® not only accelerates regulatory approval timelines but also delivers FDA-ready data that can save $25K per patient. This proactive approach fosters a culture of continuous improvement and accountability within research teams, linking innovative companies and conducting medical research across Latin America, the Balkans, and Australia. Ultimately, this collaboration leads to safer and more effective medical innovations.
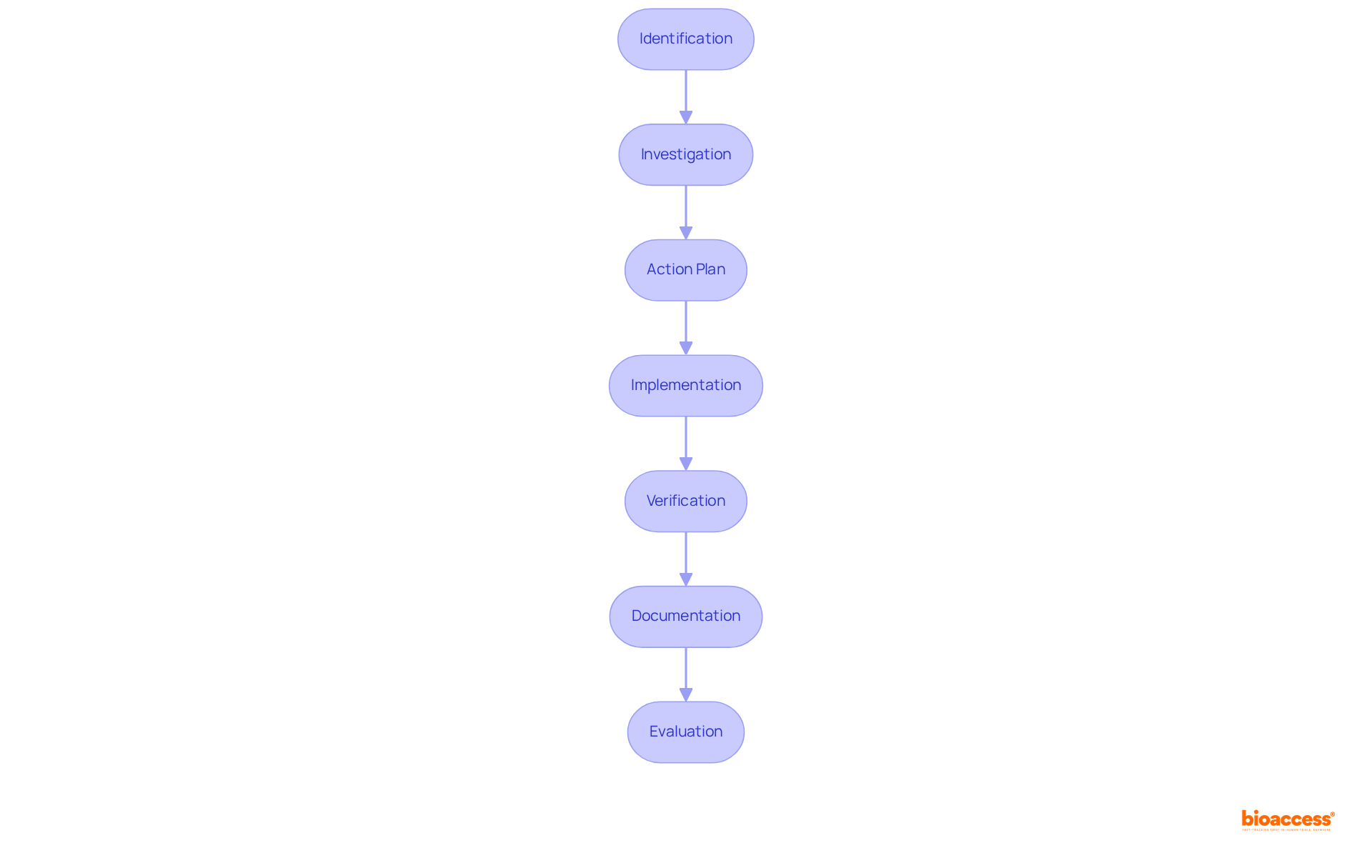
The framework for corrective and preventive actions (CAPA) is essential for maintaining quality and compliance in clinical trials. This framework consists of several critical steps:
By adhering to these steps, organizations can effectively manage non-conformities and implement corrective and preventive actions (CAPA) to enhance product quality and mitigate risks throughout the clinical research lifecycle. For instance, in a case study concerning a firm producing digital thermometers, the introduction of corrective and preventive actions resulted in extra quality evaluations and updated calibration methods, greatly enhancing product precision. Moreover, statistics indicate that organizations utilizing a robust corrective and preventive actions (CAPA) can obtain ethical approvals in as few as 4-6 weeks and recruit participants 50% quicker than conventional markets. This highlights the effectiveness of a well-executed corrective and preventive actions (CAPA). With bioaccess®'s expertise in managing trials—including Early-Feasibility, First-In-Human, Pilot Studies, and Post-Market Follow-Up Studies (PMCF)—the incorporation of corrective and preventive actions (CAPA) can further improve the speed and quality of studies, ensuring compliance and successful results.
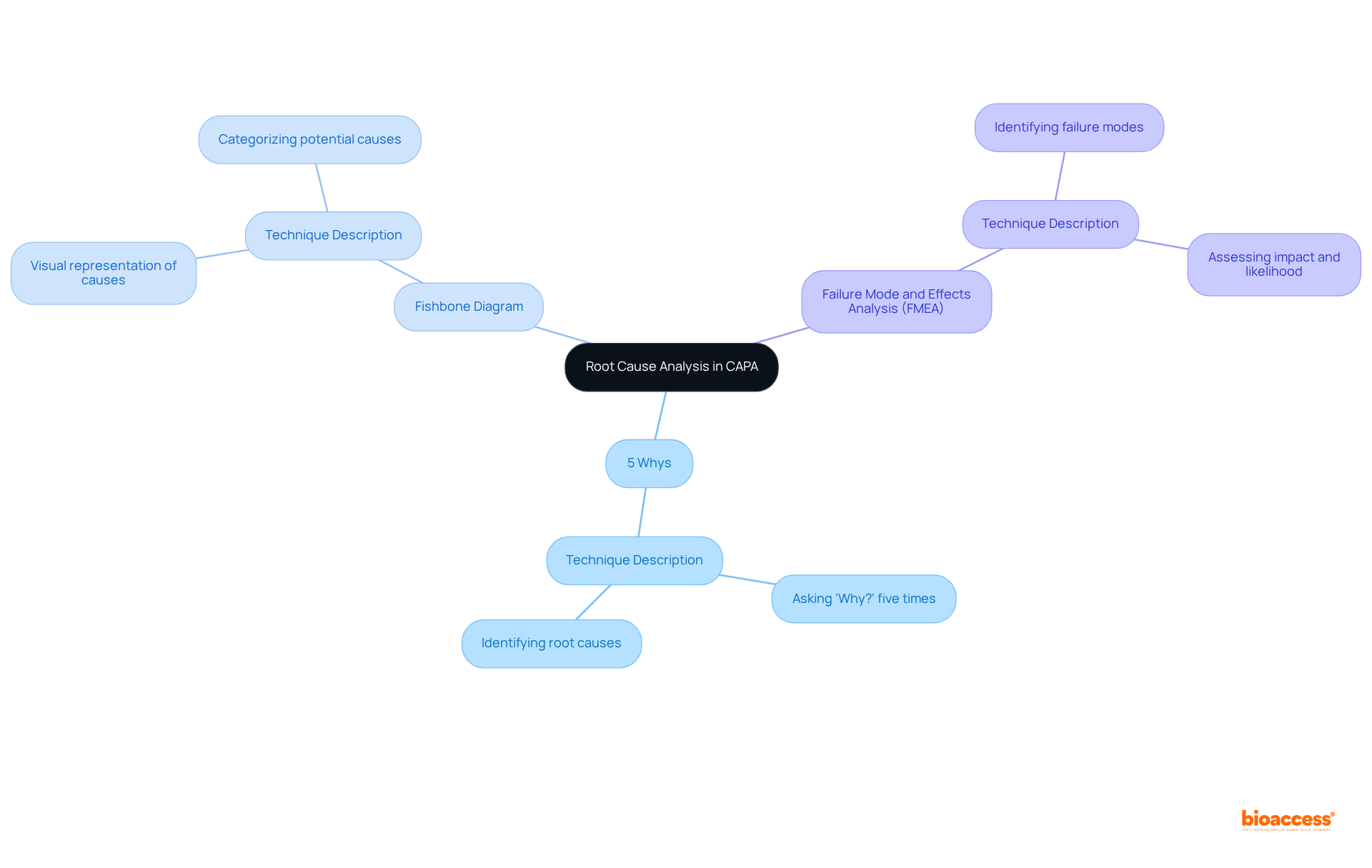
Root Cause Analysis (RCA) is a critical component of the corrective and preventive actions CAPA framework, serving to identify the underlying causes of non-conformances or adverse events. Effective RCA employs various techniques, including:
By systematically examining the contributing factors, organizations can formulate targeted corrective and preventive actions CAPA that address these root causes, thereby preventing recurrence. Integrating RCA into the quality improvement framework not only enhances the quality of clinical research but also fosters a proactive approach to risk management and compliance.
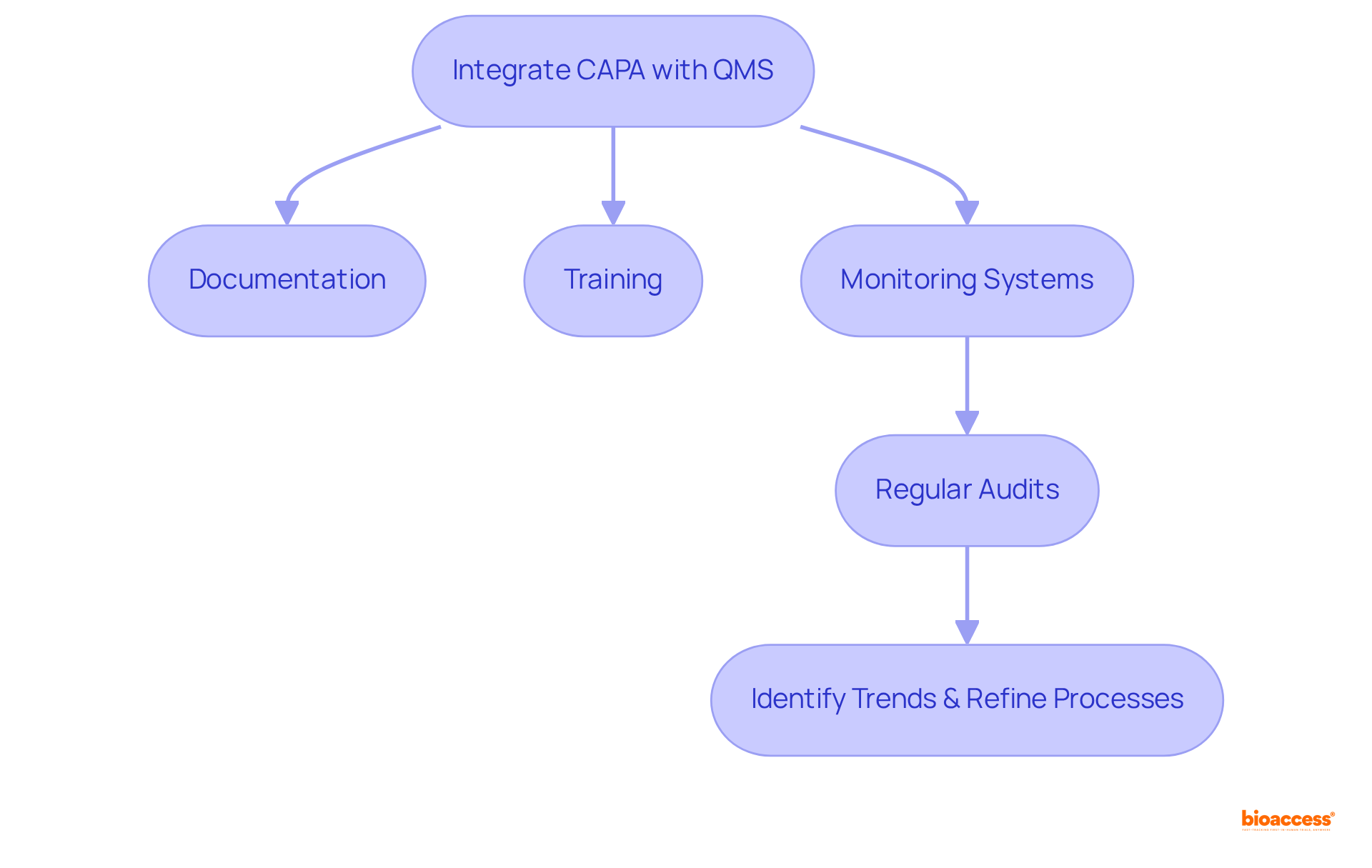
Incorporating corrective and preventive actions CAPA within Quality Management Systems (QMS) is vital for maintaining compliance and ensuring that these measures are part of a comprehensive quality framework. This integration requires the synchronization of corrective and preventive actions CAPA with quality management system documentation, training, and monitoring systems. By doing so, organizations can ensure that all team members understand the procedures for corrective and preventive actions CAPA and recognize their significance in upholding quality standards.
Furthermore, regular audits and evaluations of corrective and preventive actions CAPA within the QMS can help identify trends, refine processes, and enhance overall quality. This cohesive approach not only fortifies compliance but also cultivates a culture of continuous improvement within clinical research organizations.
Corrective and preventive actions (CAPA) are essential for upholding the integrity and quality of clinical research. By systematically identifying and addressing issues, CAPA resolves existing problems and prevents future occurrences, thereby enhancing patient safety and ensuring compliance with regulatory standards. The emphasis on CAPA in the context of clinical trials, particularly in 2025, underscores its pivotal role in fostering a culture of continuous improvement and accountability within research teams.
This article outlines the essential steps for effective CAPA implementation, including:
Each step is meticulously designed to enable organizations to manage non-conformities efficiently, ultimately leading to improved product quality and reduced risks throughout the research lifecycle. Furthermore, the integration of root cause analysis techniques significantly strengthens the CAPA process, allowing organizations to identify underlying issues and implement targeted solutions.
In conclusion, embracing a robust CAPA framework is vital for clinical research organizations aiming to enhance their quality management systems and ensure compliance. By prioritizing corrective and preventive actions, organizations can not only improve their operational efficiency but also contribute to the advancement of safer and more effective medical innovations. The call to action is clear: invest in CAPA processes today to secure a more reliable and trustworthy future in clinical research.
What are corrective and preventive actions (CAPA) in clinical research?
CAPA are systematic procedures used in medical studies to identify, examine, and rectify issues that may compromise the quality and integrity of study results. Corrective actions address existing problems, while preventive actions aim to prevent potential issues.
Why are CAPA important in clinical trials?
CAPA are critical for ensuring compliance with regulatory standards, enhancing patient safety, and upholding the credibility of research outcomes, particularly in the context of clinical trials.
What services does bioaccess® provide related to CAPA?
bioaccess® offers a comprehensive suite of services, including study design, feasibility assessments, and regulatory submissions, all essential for implementing CAPA.
How does bioaccess® facilitate the implementation of CAPA?
bioaccess® accelerates the process by securing ethical approvals within 4-6 weeks and achieving enrollment rates that are 50% faster than conventional markets, which helps in delivering FDA-ready data.
What are the financial benefits of using bioaccess® for CAPA?
By providing FDA-ready data, bioaccess® can save approximately $25,000 per patient, making the process more cost-effective.
How does the approach of bioaccess® contribute to medical research?
Their proactive approach fosters a culture of continuous improvement and accountability within research teams, linking innovative companies and conducting medical research across Latin America, the Balkans, and Australia, ultimately leading to safer and more effective medical innovations.