


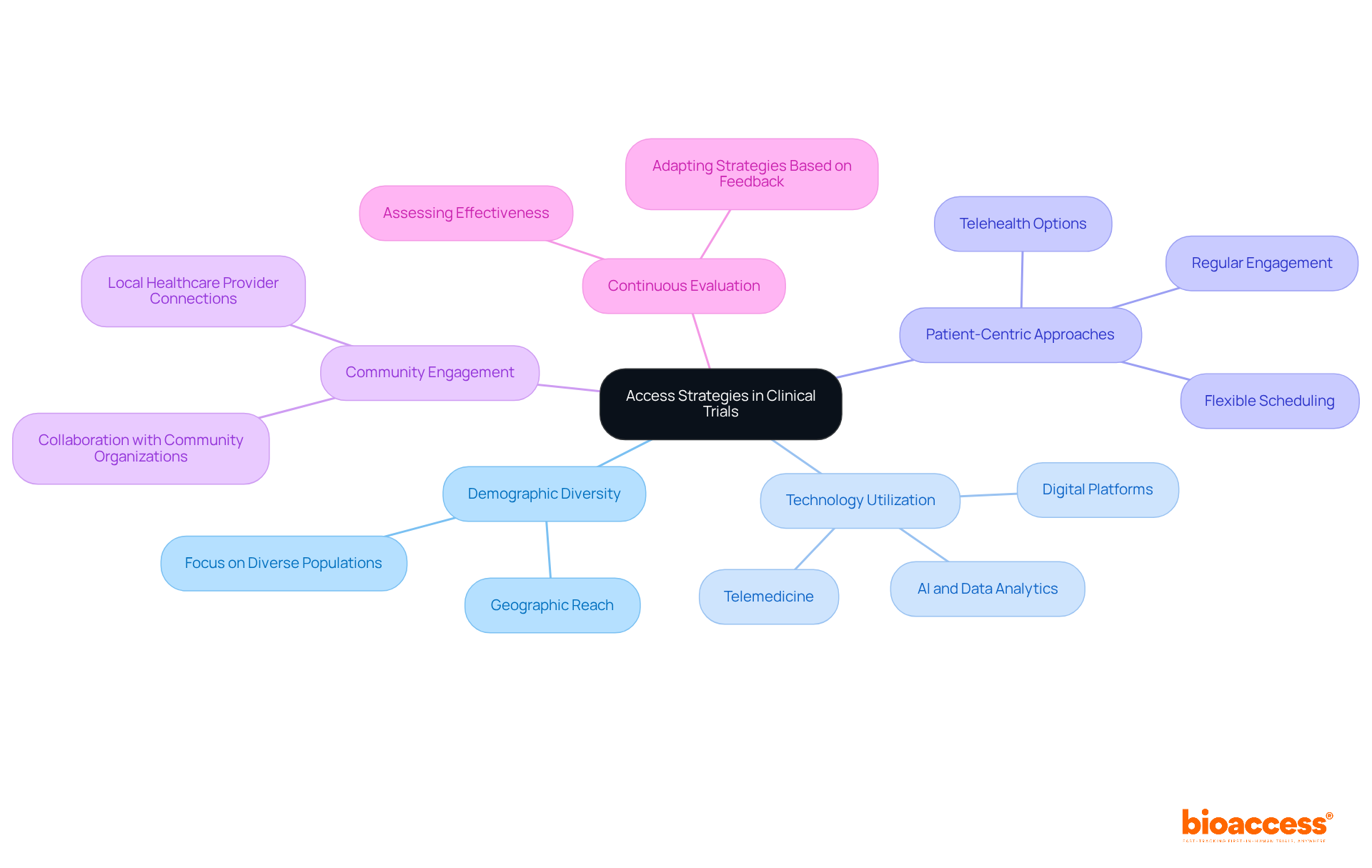
An effective access strategy for clinical trials is crucial for enhancing participant recruitment and retention. By focusing on:
Study sponsors can significantly improve their outcomes. Tailoring strategies to meet patient needs and continuously evaluating their effectiveness not only enhances representation but also leads to better research results. This approach underscores the importance of collaboration in the clinical research landscape, prompting stakeholders to consider how they can address key challenges in their own practices.
Crafting an effective access strategy for clinical trials isn’t just a procedural necessity; it’s a pivotal element that can significantly enhance participant recruitment and retention. By focusing on demographic diversity and leveraging technology, researchers can create inclusive studies that truly reflect the populations they aim to serve.
However, how can sponsors overcome the inherent challenges of participant engagement and regulatory compliance to ensure their trials not only meet but exceed enrollment goals?
This article delves into essential strategies and actionable insights needed to navigate this complex landscape successfully.
Access strategy in clinical studies is crucial for enhancing participant recruitment and retention. By establishing a clear access strategy for participant access, which focuses on demographic diversity and geographic reach, studies can ensure they represent the groups they aim to assist.
Utilizing technology is another key component. Digital platforms, including social media and dedicated trial websites, can significantly boost visibility. With over 3 billion active users on platforms like Facebook and Instagram, effective online engagement can enhance recruitment efforts dramatically.
Moreover, adopting patient-centric approaches is essential. Strategies that prioritize patient needs, such as flexible scheduling and telehealth options, not only increase participation rates but also address the 30% dropout rate frequently observed in clinical studies.
Community engagement plays a vital role as well. Establishing connections with local healthcare providers and community organizations cultivates trust and raises awareness about the study. By interacting with these organizations, potential participants can be connected to research opportunities.
Finally, continuous evaluation of the access strategy is necessary. Regularly assessing the effectiveness of these approaches and adapting them based on participant feedback and enrollment data is crucial for improving recruitment and ensuring studies meet their enrollment goals.
By understanding and applying these strategies, study sponsors can create a more inclusive and efficient participant selection process, ultimately leading to enhanced study outcomes.
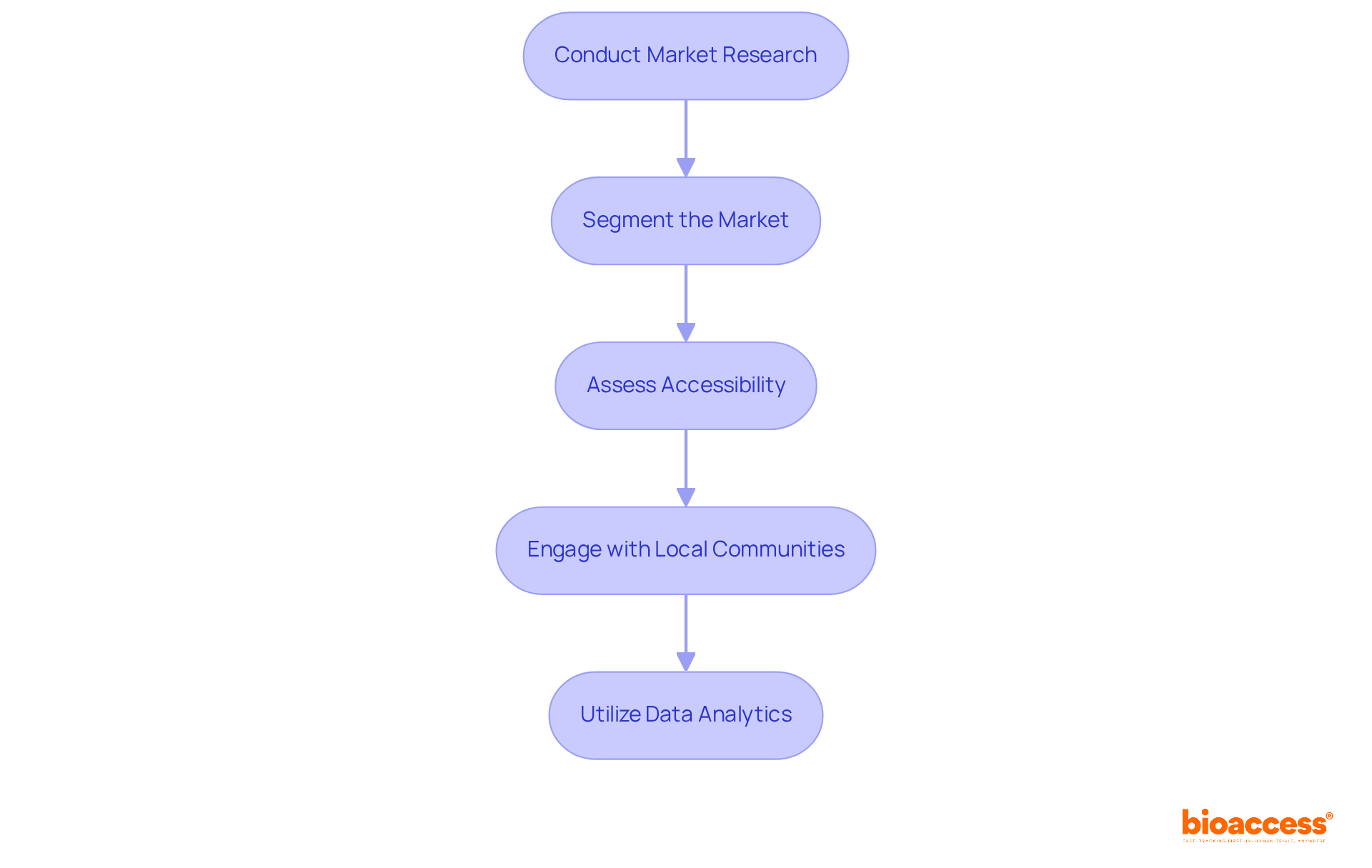
Identifying target markets for research studies is crucial for enhancing recruitment efforts and ensuring a diverse participant pool. This systematic approach not only improves the quality of research but also addresses significant gaps in representation. Here are the key steps to follow:
Conduct Market Research: Analyze demographic data, disease prevalence, and treatment gaps to pinpoint potential participant populations. Understanding the distinct experiences of patients with specific conditions is essential, as research studies often reflect biased demographics, primarily including white, male patients. Notably, individuals of color make up approximately 39% of the U.S. population, underscoring the urgent need for greater diversity in research studies.
Segment the Market: Divide the target population into segments based on characteristics such as age, gender, and health status. This tailored approach enables more effective recruitment strategies by incorporating an access strategy that resonates with various demographic groups.
Assess Accessibility: Evaluate the geographical distribution of potential participants and consider logistical factors that may impact their ability to participate. Accessibility poses a significant barrier; an access strategy that addresses patient distrust and low awareness can help to enhance enrollment. For instance, doctors refer a median of five patients to research studies each year, which accounts for less than 0.2% of their patient volume. This highlights the importance of involving healthcare providers in the access strategy for recruitment.
Engage with Local Communities: Build partnerships with local healthcare providers and organizations to gain insights into community needs and preferences. Involving trusted sources of information, such as doctors and nurses, can significantly influence patient decisions to participate in research studies. The critical role that clinical care providers play in determining eligibility and facilitating patient engagement is vital for improving referral rates.
Utilize Data Analytics: Employ data analysis tools to monitor recruitment trends and adjust strategies accordingly. Real-world data can provide enhanced demographic estimates for disease populations, aiding informed decisions regarding enrollment efforts. This approach aligns with the necessity for comprehensive market research plans that accurately represent the diversity of affected populations.
By effectively identifying target markets, research sponsors can enhance their access strategy for recruitment, ultimately leading to more representative and successful studies. For example, the implementation of a new assessment framework in rheumatoid arthritis and stroke studies has demonstrated the effectiveness of focused market research strategies, resulting in improved demographic representation and a deeper understanding of treatment efficacy across various groups.
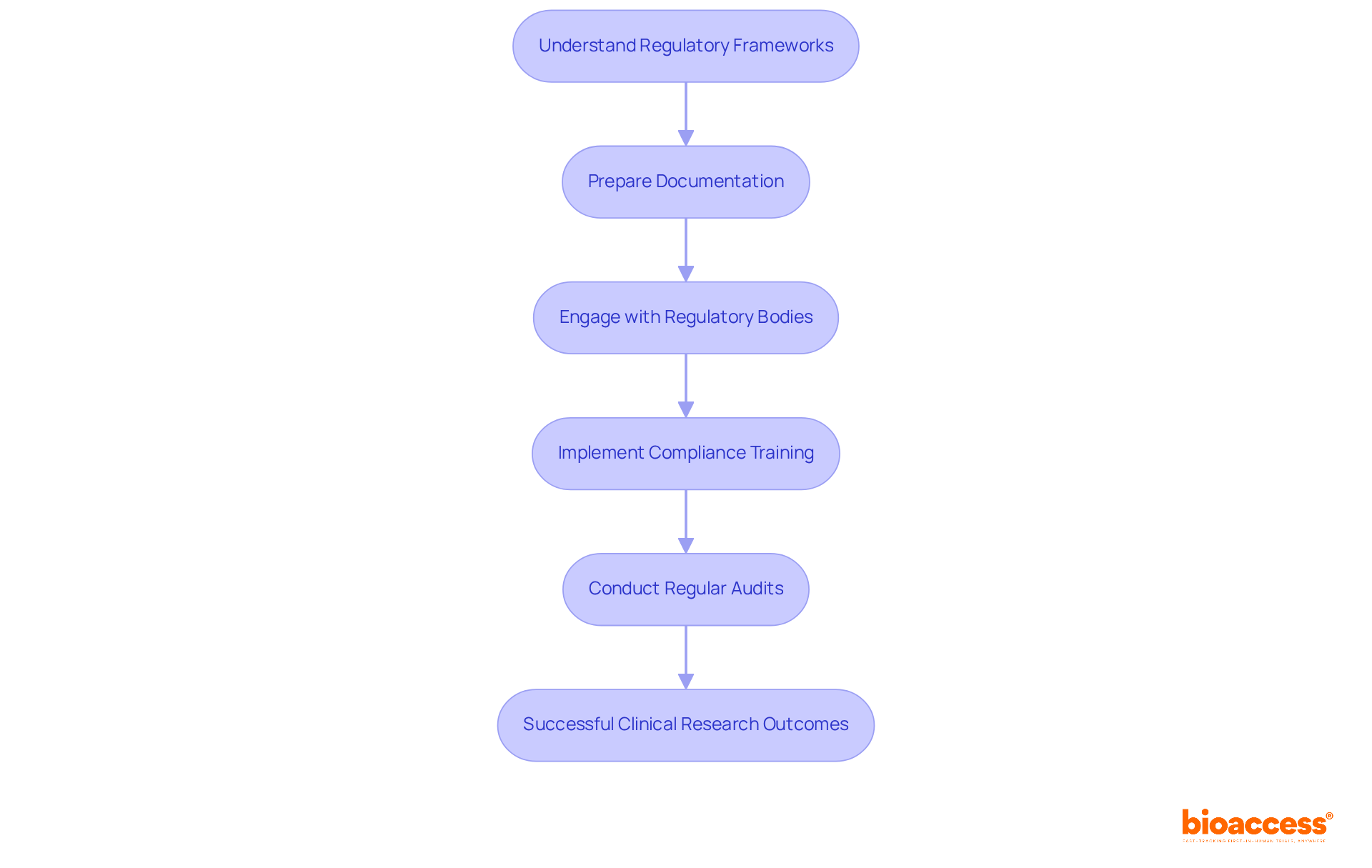
Navigating regulatory requirements is essential for the success of clinical research. Understanding the regulatory frameworks is the first step. Familiarize yourself with local and international regulations, including FDA guidelines and ICH GCP standards. Staying updated on these evolving regulations is crucial to address new challenges in clinical research.
Next, prepare your documentation meticulously. Ensure that all necessary materials, such as study protocols and informed consent forms, align with regulatory standards. Proper documentation not only facilitates smoother approvals but also enhances the credibility of your study.
Engaging with regulatory bodies is another vital step. Establish proactive communication with relevant authorities early in the study design process. This engagement helps identify and address potential concerns, fostering a collaborative relationship that can expedite the approval process.
Implementing compliance training is crucial as well. Provide comprehensive training for all team members on regulatory requirements and ethical considerations. Effective training is essential; studies indicate that only 13% of research professionals fully adhere to training protocols. This highlights the necessity for engaging and impactful training techniques that resonate with participants.
Lastly, conduct regular audits. Implement a robust system for ongoing monitoring to ensure compliance. Regular assessments not only help identify issues promptly but also reinforce a culture of accountability and continuous improvement within the research team.
By effectively navigating these regulatory requirements, research sponsors can minimize risks, enhance the integrity of their studies, and ultimately contribute to the successful advancement of medical innovations.
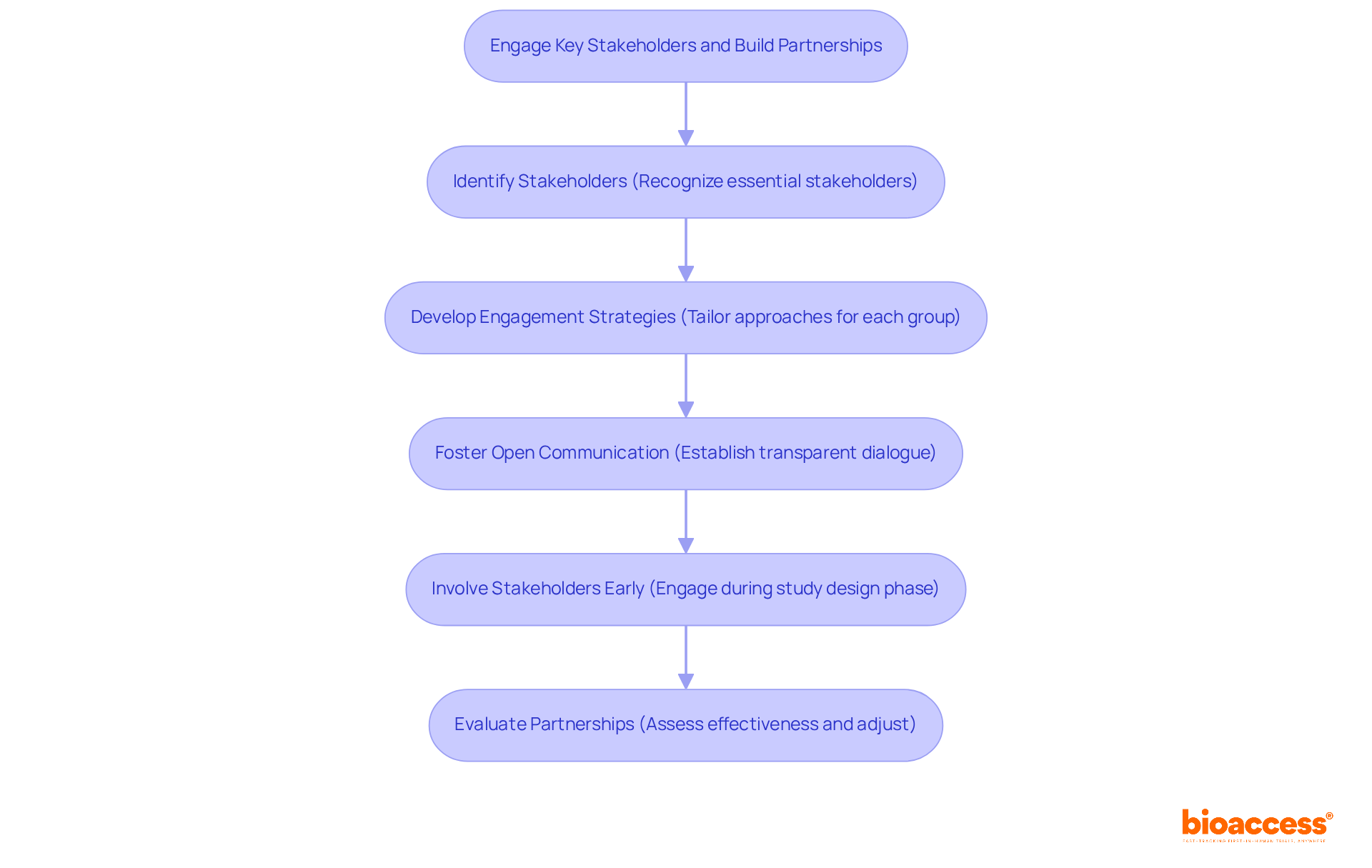
To effectively engage key stakeholders and cultivate successful partnerships in clinical research, it’s essential to implement an access strategy that resonates with their interests and concerns.
Identify Stakeholders: Recognizing essential stakeholders—patients, healthcare providers, regulatory agencies, and community organizations—is crucial for study success. Their involvement can significantly influence outcomes.
Develop Engagement Strategies: Tailoring engagement approaches for each stakeholder group fosters a sense of ownership and commitment. Addressing their unique interests ensures that they feel valued and invested in the research process.
Foster Open Communication: Establishing transparent dialogue with stakeholders is vital for building trust and promoting collaboration. As Karen Marder emphasizes, participant trust is key to achieving enrollment objectives, making open lines of communication indispensable.
Involve Stakeholders Early: Engaging stakeholders during the study design phase allows for the incorporation of their insights, enhancing buy-in and support. This proactive approach can lead to improved recruitment outcomes. Notably, over two-thirds of sites fail to meet original patient enrollment targets, underscoring the importance of early involvement. This principle is particularly relevant in bioaccess™'s collaboration with Welwaze Medical Inc. for the Celbrea® medical device launch, where the access strategy and stakeholder engagement are pivotal for success in the Colombian market.
Evaluate Partnerships: Continuously assessing the effectiveness of collaborations is essential. Making necessary adjustments strengthens cooperation and ensures alignment with project objectives. With 85% of medical studies facing challenges with patient retention, ongoing involvement is critical. The obstacles encountered by medical device startups, including regulatory barriers and competition, further highlight the significance of these approaches.
By actively involving stakeholders, study sponsors can create a supportive atmosphere that greatly enhances recruitment and retention efforts. This method is particularly significant given that almost 80% of research studies do not achieve enrollment goals, emphasizing the need for an effective access strategy for collaboration. Additionally, the average dropout rate across all clinical trials stands at 30%, reinforcing the importance of maintaining stakeholder engagement throughout the trial process.
Crafting a robust access strategy is essential for enhancing the effectiveness of clinical trials. By concentrating on participant recruitment and retention through targeted approaches, studies can ensure they encompass diverse populations and address the needs of those they aim to benefit. An effective access strategy not only broadens the participant pool but also significantly contributes to the overall success and integrity of clinical research.
The article outlines several key components vital for developing an effective access strategy. These include:
Each element plays a crucial role in fostering an inclusive environment that encourages participation and supports the integrity of clinical trials. Moreover, the importance of stakeholder engagement and building partnerships cannot be overstated, as these relationships are instrumental in improving recruitment and retention rates.
In light of these insights, it is crucial for research sponsors to prioritize the development and implementation of effective access strategies. By doing so, they not only enhance the quality of their studies but also contribute to more equitable healthcare outcomes. Emphasizing collaboration and continuous evaluation will ensure that clinical trials are not only representative but also capable of delivering meaningful results that can drive medical innovations forward.
What is the importance of access strategies in clinical trials?
Access strategies in clinical trials are crucial for enhancing participant recruitment and retention, ensuring that studies represent the demographic diversity and geographic reach of the groups they aim to assist.
How can technology improve participant recruitment in clinical trials?
Technology, particularly digital platforms like social media and dedicated trial websites, can significantly boost visibility and engagement, thereby enhancing recruitment efforts. With over 3 billion active users on platforms like Facebook and Instagram, effective online engagement is key.
What patient-centric approaches can increase participation rates in clinical trials?
Patient-centric approaches that prioritize patient needs, such as flexible scheduling and telehealth options, can increase participation rates and help address the common 30% dropout rate in clinical studies.
Why is community engagement important in clinical trials?
Community engagement is vital as it helps establish connections with local healthcare providers and organizations, cultivating trust and raising awareness about the study. This interaction can connect potential participants to research opportunities.
How should access strategies be evaluated in clinical trials?
Continuous evaluation of access strategies is necessary. Regularly assessing the effectiveness of approaches and adapting them based on participant feedback and enrollment data is crucial for improving recruitment and meeting enrollment goals.
What is the overall goal of implementing these access strategies in clinical trials?
The overall goal is to create a more inclusive and efficient participant selection process, which ultimately leads to enhanced study outcomes.