

The article emphasizes the critical steps involved in creating a clinical study timeline, highlighting its vital role in organizing research, ensuring regulatory compliance, managing risks, and facilitating effective communication among stakeholders. A well-structured timeline is not just a tool; it’s a cornerstone of successful clinical trials. By detailing the phases of study, estimating durations, incorporating necessary buffers, and drafting a comprehensive timeline document, the article illustrates how a systematic approach can significantly boost the success rates of clinical trials.
In the ever-evolving Medtech landscape, understanding the intricacies of clinical study timelines is essential. This structured approach addresses key challenges faced by researchers and stakeholders alike. The importance of collaboration cannot be overstated; it is through teamwork that we can navigate the complexities of clinical research effectively.
Ultimately, the article serves as a call to action for researchers to adopt these practices. By implementing a well-defined timeline, they can enhance their research efforts and contribute to the advancement of medical science. The path to successful clinical trials begins with a clear, organized approach.
Crafting a clinical study timeline is not just a logistical exercise; it serves as a crucial blueprint that can dictate the success or failure of a research project. A well-structured timeline organizes tasks, ensures compliance with regulatory standards, and enhances communication among stakeholders. Yet, with approximately 80% of clinical trials struggling to meet enrollment goals, the real challenge lies in effectively managing timelines to mitigate risks and optimize outcomes.
How can researchers navigate these complexities to create a timeline that truly drives their study forward?
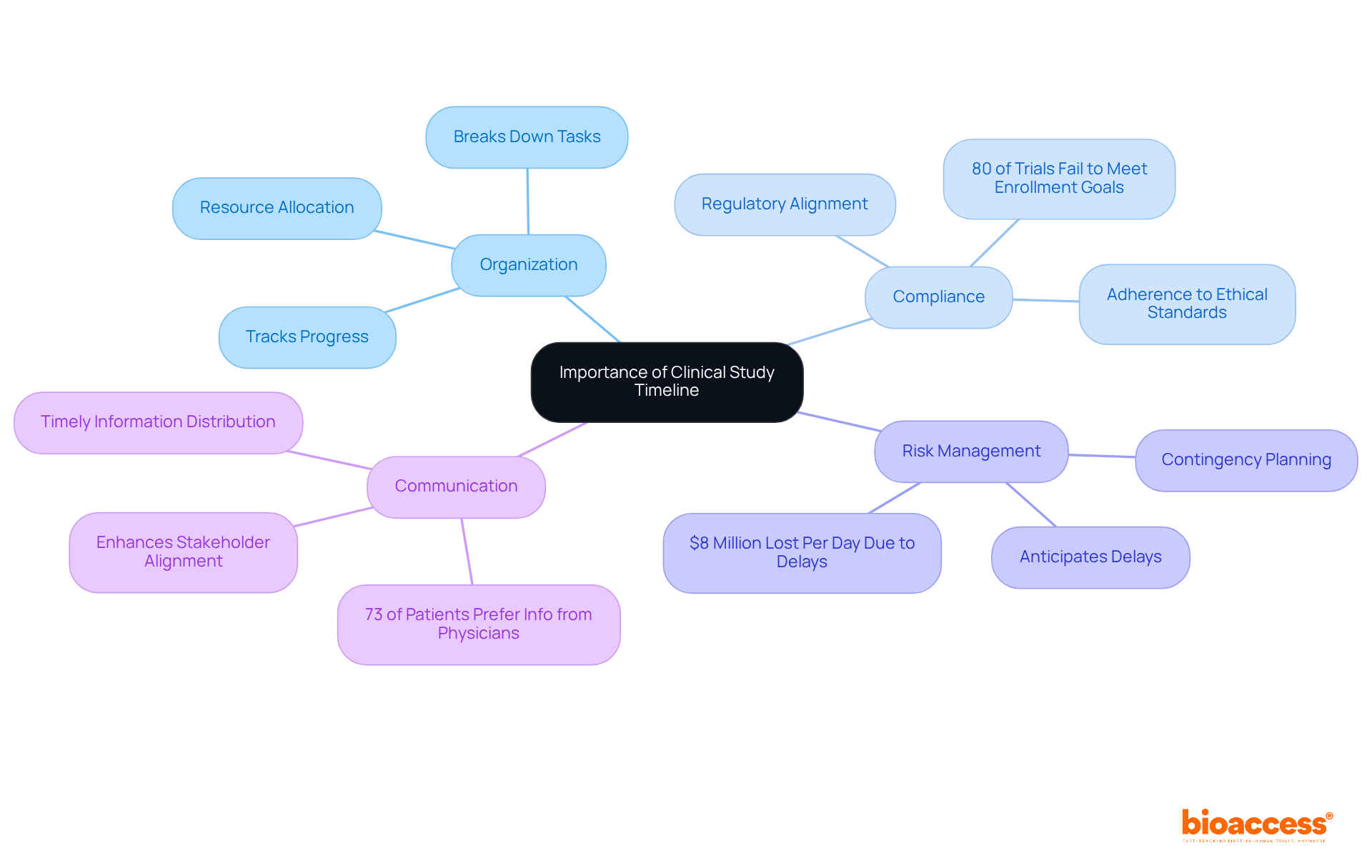
A clinical study timeline is essential for guiding the entire research project schedule, outlining each stage from initiation to completion. Its significance is multifaceted:
Organization: A well-structured timeline breaks down the research into manageable tasks, making it easier to track progress and allocate resources effectively. This organized approach is crucial, especially considering that the average length of Phase 3 studies has increased by about one year over the last decade, which underscores the need for a meticulous clinical study timeline. bioaccess® offers comprehensive management services for research studies, including feasibility assessments, site selection, study setup, and project oversight, streamlining this process.
Compliance with regulatory bodies requires a detailed clinical study timeline to ensure adherence to ethical standards and protocols. This compliance is vital, as approximately 80% of clinical trials fail to meet initial enrollment goals, often due to insufficient planning and oversight. bioaccess® provides evaluation and input on study documents to align with country requirements, ensuring that schedules meet regulatory expectations.
Risk Management: By anticipating potential delays and challenges, a schedule helps in crafting contingency plans, significantly reducing the risk of project overruns. For example, delays in patient enrollment can cost drug discovery companies up to $8 million per day, highlighting the financial stakes involved. bioaccess®'s project management and monitoring services effectively mitigate these risks.
Communication: A clear timeline enhances communication among stakeholders, ensuring everyone is aligned on expectations and deadlines. Efficient communication is crucial, as research shows that 73% of patients prefer learning about research opportunities from their physicians, emphasizing the importance of timely and accurate information distribution. bioaccess® prioritizes communication in its study setup and management processes, ensuring all parties are informed and engaged.
In conclusion, a well-defined clinical study timeline is critical for the successful execution of trials, as it influences every aspect from recruitment to data analysis and ultimately affects research success rates. By leveraging the specialized services of bioaccess®, including trial setup and project management, you can accelerate trials and enhance overall outcomes.
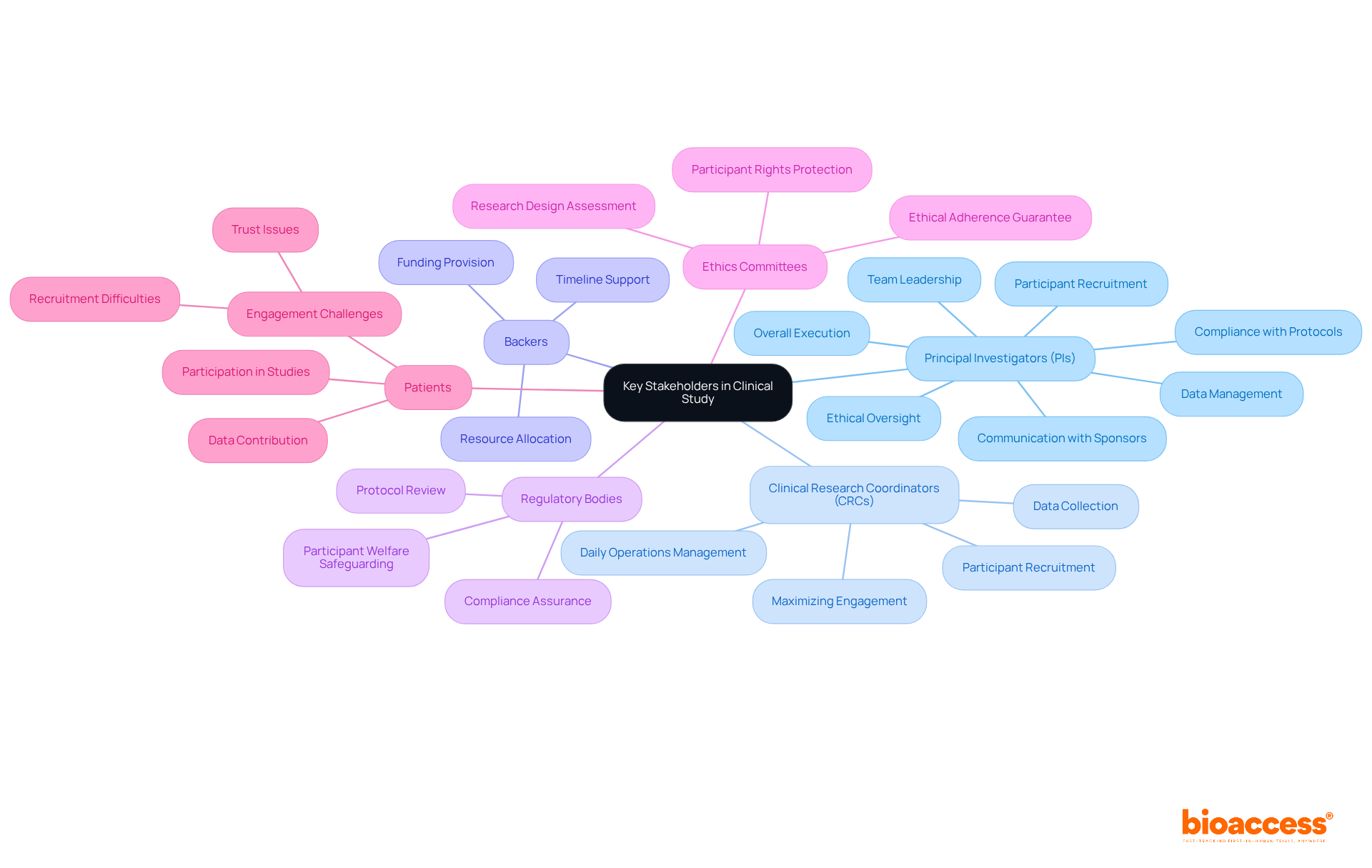
Key stakeholders in a clinical study play pivotal roles that significantly impact research outcomes:
Principal Investigators (PIs): As the cornerstone of clinical research, PIs are responsible for the overall execution of the project, ensuring compliance with protocols and regulations. Their leadership is crucial, overseeing participant recruitment, data management, and adherence to ethical standards. Effective PIs enhance research outcomes by managing multidisciplinary teams and maintaining communication with sponsors and regulatory bodies.
Clinical Research Coordinators (CRCs): CRCs manage the daily operations of the project, including participant recruitment and data collection. Their role is essential in ensuring that the research operates efficiently and that participant engagement is maximized.
Backers: Typically pharmaceutical or biotech firms, backers supply the essential funding and resources for the research. Their investment is critical for the successful execution of the clinical study timeline for clinical trials.
Regulatory bodies ensure that the research adheres to ethical and legal standards by reviewing protocols and the clinical study timeline, which helps safeguard participant welfare and research integrity.
Ethics Committees: Tasked with safeguarding participant rights, ethics committees assess the research's design and execution to guarantee ethical adherence.
Patients: The ultimate focus of the study, patient participation is crucial for data collection and achieving meaningful outcomes. However, recruitment difficulties are substantial, with approximately 80% of clinical studies encountering delays or closures due to recruitment problems. Engaging patients effectively can mitigate these challenges and enhance trial success.
By clearly defining these roles and responsibilities, the research can proceed smoothly, with each stakeholder understanding their contributions to the overall success of the project. The influence of PIs on research outcomes is significant; they not only guarantee adherence but also promote innovation and ethical behavior, which are vital for progressing medical knowledge.
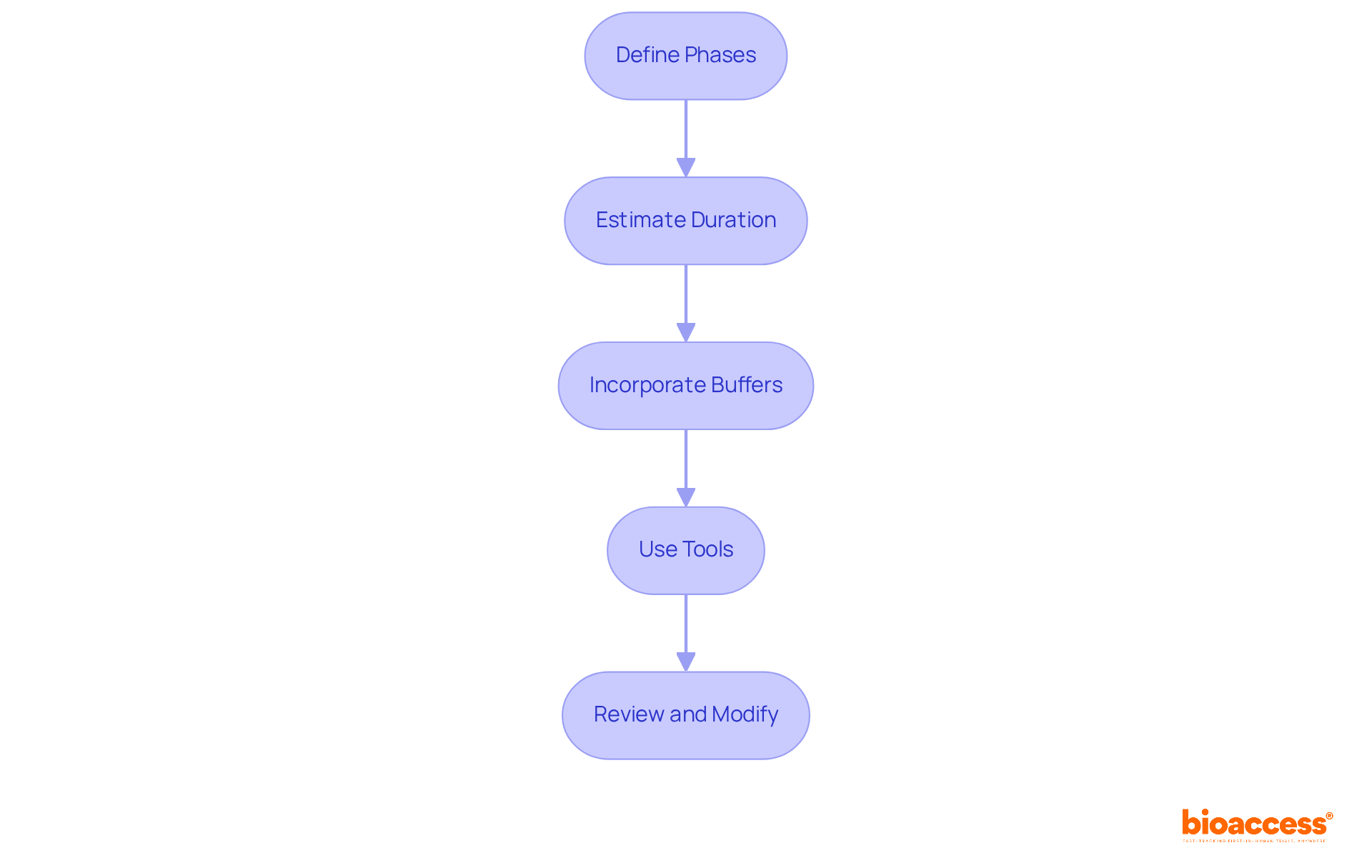
To establish realistic timelines for each phase of a clinical study, it’s essential to follow a structured approach:
By following these steps, you can develop a practical schedule that not only improves the chances of completing the research on time but also aligns with the clinical study timeline to ensure it stays within budget.
To create an effective clinical study timeline document, follow these essential guidelines:
By developing a comprehensive clinical study timeline, you establish a structured framework for the study, promoting effective communication and accountability among all involved parties.
A meticulously crafted clinical study timeline is the backbone of successful research projects, guiding each phase from inception to completion. By prioritizing organization, compliance, risk management, and communication, a well-defined timeline not only enhances the efficiency of clinical trials but also significantly improves the likelihood of achieving research objectives.
Throughout this article, we explored key aspects of creating an effective clinical study timeline. The importance of identifying stakeholders and their roles was emphasized, as each participant—from Principal Investigators to patients—plays a critical role in the study's success. Establishing realistic timelines for each phase, incorporating buffers for unforeseen delays, and drafting a comprehensive timeline document with clear milestones are essential steps that ensure smooth project execution.
Ultimately, the significance of a clinical study timeline cannot be overstated. It is a vital tool that fosters collaboration among stakeholders, mitigates risks, and aligns research efforts with regulatory standards. As the landscape of clinical research continues to evolve, investing time in developing a robust timeline will not only streamline processes but also enhance outcomes, paving the way for advancements in medical knowledge and patient care.
What is the purpose of a clinical study timeline?
A clinical study timeline serves as a guide for the entire research project schedule, outlining each stage from initiation to completion. It helps in organizing tasks, tracking progress, and allocating resources effectively.
Why is organization important in a clinical study timeline?
Organization is crucial because it breaks down research into manageable tasks, making it easier to track progress. This is especially important given that the average length of Phase 3 studies has increased by about one year over the last decade.
How does a clinical study timeline ensure compliance with regulatory bodies?
A detailed clinical study timeline is necessary to ensure adherence to ethical standards and protocols, which is vital for compliance. Insufficient planning and oversight can lead to failures in meeting initial enrollment goals in clinical trials.
What role does risk management play in a clinical study timeline?
Risk management involves anticipating potential delays and challenges, allowing for the creation of contingency plans. This significantly reduces the risk of project overruns, as delays in patient enrollment can incur substantial costs.
How does a clinical study timeline enhance communication among stakeholders?
A clear timeline improves communication by ensuring that all stakeholders are aligned on expectations and deadlines. Efficient communication is essential for timely and accurate information distribution, particularly regarding patient recruitment.
What services does bioaccess® provide to assist with clinical study timelines?
bioaccess® offers comprehensive management services for research studies, including feasibility assessments, site selection, study setup, project oversight, evaluation of study documents, and project management to mitigate risks.
What impact does a well-defined clinical study timeline have on research outcomes?
A well-defined clinical study timeline influences every aspect of the research process, from recruitment to data analysis, ultimately affecting research success rates and trial execution.