


Creating a clinical trial risk management plan (RMP) is not merely a regulatory requirement; it stands as a crucial strategy that can profoundly impact the success of clinical research in Romania. By effectively identifying and mitigating risks, researchers enhance participant safety and uphold the integrity of their data. Yet, the challenge lies in navigating the intricate regulatory landscape while continuously adapting to emerging findings and potential threats.
How can researchers develop a robust RMP that not only meets compliance standards but also cultivates a culture of safety and trust within their clinical trials? This question is pivotal, as a well-structured RMP not only safeguards participants but also fortifies the research process itself.
A Risk Management Plan (RMP) is crucial in clinical trials, serving to identify, characterize, and mitigate threats associated with the study. In Romania, the clinical trial risk management plan (RMP) in Romania goes beyond mere regulatory compliance; it establishes a strategic framework that prioritizes participant safety and maintains the integrity of collected data. By proactively addressing potential risks, researchers can enhance the credibility of their findings and facilitate the regulatory approval process. An effective RMP not only improves decision-making throughout the study but also leads to better patient outcomes and successful product development. The implementation of a robust RMP fosters a culture of security and optimizes resource utilization, ultimately contributing to the overall success of clinical research initiatives.

In Romania, the development of a clinical trial risk management plan (RMP) in Romania is crucial for ensuring the safety and efficacy of medicinal products. Governed by the guidelines established by the National Agency for Medicines and Medical Devices (NAMMD) and the European Medicines Agency (EMA), these regulations are anchored in the Commission Implementing Regulation No 520/2012. This regulation outlines the necessary format and content for RMPs, emphasizing the importance of compliance.
Key components of an effective RMP include:
It’s essential to not only adhere to these guidelines but also to regularly update the clinical trial risk management plan (RMP) in Romania to incorporate new data and findings throughout the clinical trial process. How often do you evaluate your RMP against these evolving standards? A comprehensive understanding of these regulatory requirements will significantly enhance the effectiveness and adherence of your RMP.
Ultimately, this diligence aids in the security and efficacy of the medicinal products under investigation. By prioritizing a robust clinical trial risk management plan (RMP) in Romania, you position your clinical research efforts for success, ensuring that safety remains at the forefront of your initiatives.

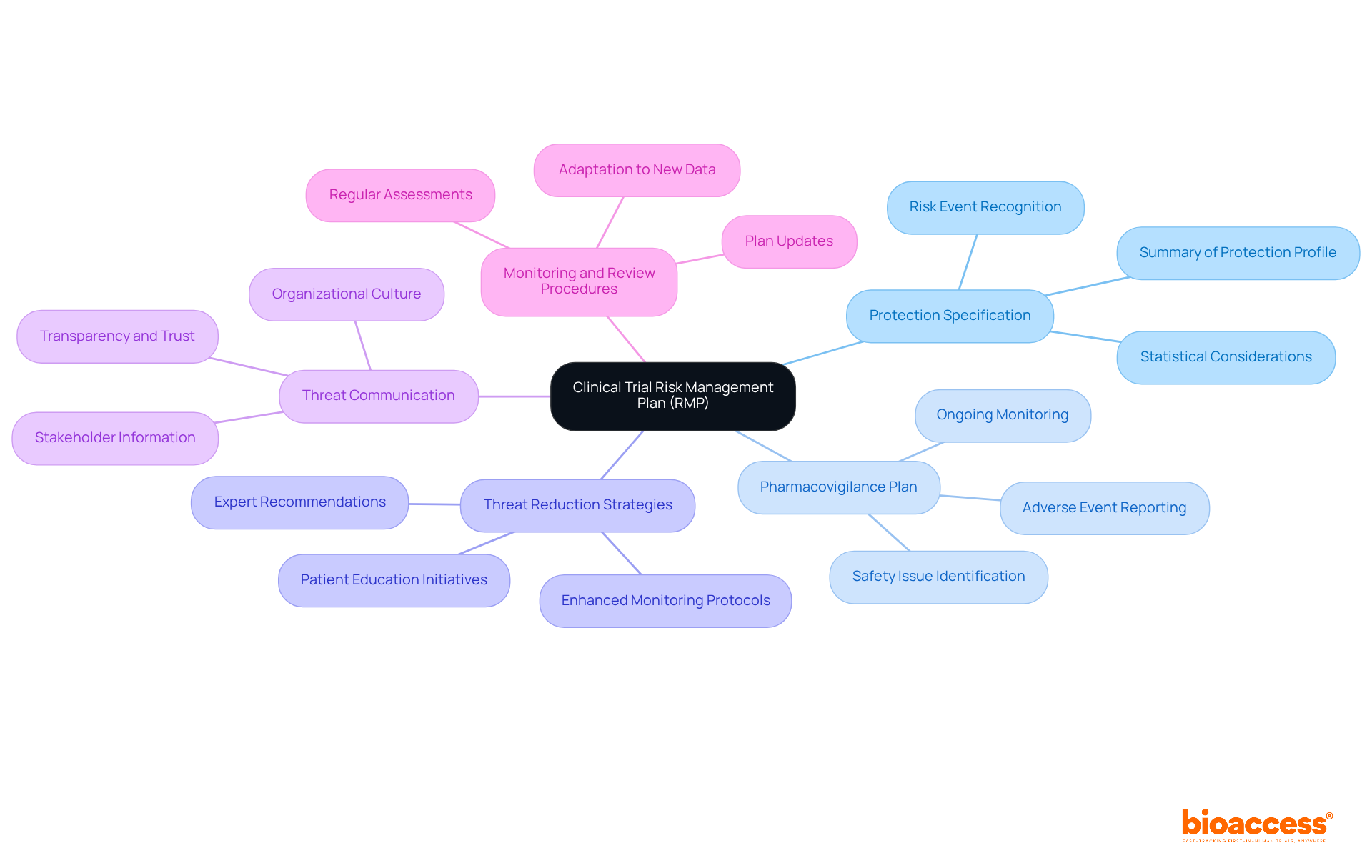
An effective clinical trial risk management plan (RMP) in Romania is crucial for ensuring the safety and efficacy of clinical trials. It must encompass several essential components that not only comply with regulatory requirements but also enhance the overall testing environment.
Protection Specification: This section provides a comprehensive summary of the investigational product's protection profile, detailing known risks and potential adverse effects. Recognizing risk events, often viewed as binary occurrences-like specific toxicities within a defined timeframe-is essential. Statistics reveal that small values of the concentration parameter in the beta-binomial model indicate a weak prior, underscoring the necessity for robust protective specifications.
Pharmacovigilance Plan: This outlines strategies for the ongoing monitoring and reporting of adverse events. A strong pharmacovigilance plan is vital for promptly addressing concerns, thereby enhancing patient well-being and trial integrity. Continuous monitoring is key to identifying critical safety issues, as recent studies have shown.
Threat Reduction Strategies: This includes specific actions aimed at mitigating identified hazards. Examples may involve enhanced monitoring protocols or patient education initiatives designed to lower the likelihood of adverse events. Expert opinions emphasize that effective threat reduction is essential for maintaining trial integrity.
Threat Communication: Clear communication strategies are vital for informing all stakeholders about potential hazards and the measures in place to address them. Effective communication fosters transparency and trust among participants, regulatory bodies, and sponsors. This aligns with the notion that uncertainty management should be ingrained in the organizational culture.
Monitoring and Review Procedures: Regular assessments of the RMP's effectiveness are necessary to adapt to new data or findings. Ongoing supervision ensures that the plan remains relevant and effective in addressing challenges throughout the trial. The importance of keeping the management plan updated is critical for effective project administration, as highlighted by industry specialists.
The significance of Safety Specifications in the clinical trial risk management plan (RMP) in Romania cannot be overstated. They form the foundation for risk assessment and management, guiding the development of the pharmacovigilance plan and informing risk minimization strategies. Expert insights, including those from Peter L. Bernstein, stress that a clearly defined safety specification is vital for enhancing the quality and reliability of safety data in clinical studies. By integrating these components, the clinical trial risk management plan (RMP) in Romania not only complies with regulatory standards but also significantly enhances the safety of the testing environment.
Effective implementation of the clinical trial risk management plan (RMP) in Romania is crucial throughout the clinical trial process. This begins with comprehensive training for the research team on the clinical trial risk management plan (RMP) in Romania's components, ensuring that all staff members are aware of their specific roles in risk management. Regular monitoring is essential to assess the RMP's effectiveness, which includes several key activities:
By proactively overseeing and tracking the clinical trial risk management plan (RMP) in Romania, researchers can successfully reduce uncertainties and ensure that the study complies with regulatory standards. This structured approach not only enhances the safety of participants but also supports the integrity of the clinical trial risk management plan (RMP) in Romania within the clinical research process.
Bioaccess offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, ensuring a robust framework for effective risk management.

A well-structured Risk Management Plan (RMP) is essential in the realm of clinical trials, especially in Romania. It acts as a crucial tool that not only ensures compliance with regulatory mandates but also prioritizes participant safety and the integrity of research data. By proactively identifying and mitigating potential risks, researchers can enhance the credibility of their findings and streamline the regulatory approval process. This ultimately leads to improved patient outcomes and successful product development.
Throughout this article, we outlined the key components of an effective RMP, emphasizing the importance of:
Each of these elements plays a vital role in fostering a secure testing environment and ensuring that trials adapt to new data and challenges. Regular updates and assessments of the RMP are crucial to maintain its relevance and effectiveness in addressing the evolving landscape of clinical research.
In conclusion, the significance of implementing a comprehensive clinical trial risk management plan in Romania cannot be overstated. It not only safeguards the well-being of participants but also enhances the overall quality of clinical research. By committing to these best practices, researchers can contribute to the advancement of medical science while upholding the highest standards of ethics and safety in clinical trials. Embracing a proactive approach to risk management is not just beneficial; it is essential for the success and integrity of clinical research initiatives.
What is a Risk Management Plan (RMP) in clinical trials?
A Risk Management Plan (RMP) in clinical trials is a strategic framework that identifies, characterizes, and mitigates threats associated with the study, ensuring participant safety and data integrity.
Why is an RMP important in clinical trials in Romania?
In Romania, an RMP goes beyond regulatory compliance by prioritizing participant safety, maintaining data integrity, and enhancing the credibility of findings, which facilitates the regulatory approval process.
How does an effective RMP impact decision-making in clinical trials?
An effective RMP improves decision-making throughout the study by proactively addressing potential risks, leading to better patient outcomes and successful product development.
What are the benefits of implementing a robust RMP in clinical research?
Implementing a robust RMP fosters a culture of security, optimizes resource utilization, and contributes to the overall success of clinical research initiatives.