


The article emphasizes the effective creation of a Mexico labeling NOM design package, essential for compliance with the official labeling standards in Mexico. It delineates crucial components such as:
The importance of adhering to the Norma Oficial Mexicana (NOM) regulations is underscored, as it is vital for ensuring consumer safety and avoiding penalties. This focus on compliance not only safeguards consumers but also reinforces the credibility of businesses in the Medtech landscape.
Navigating the intricate landscape of product labeling in Mexico presents a significant challenge for businesses striving to comply with the Norma Oficial Mexicana (NOM) standards. With the recent implementation of Phase 2 of NOM-051, which intensifies the requirements for nutritional information and labeling clarity, companies face an urgent need to adapt. This guide serves as a comprehensive roadmap for developing a NOM-compliant labeling design package, ensuring that brands not only meet regulatory demands but also cultivate consumer trust. However, with evolving regulations and potential pitfalls lurking at every turn, how can businesses effectively streamline their labeling processes while avoiding costly compliance issues?
The Norma Oficial Mexicana (NOM) standards establish the Mexico labeling NOM design package as compulsory regulations for the labeling of products sold in Mexico. These standards ensure that consumers receive accurate and clear information about their purchases. Key aspects of NOM standards include:
Understanding the Mexico labeling NOM design package standards is crucial to avoid compliance issues and potential penalties. As of October 1, 2023, the implementation of Phase 2 of NOM-051 will enhance product information requirements, focusing on the evaluation of complementary nutritional details for prepackaged foods and non-alcoholic beverages. This phase will continue until September 30, 2025. Companies must review and update their labels, specifically the Mexico labeling NOM design package, to align with these new standards and avoid fines. For assistance, TC Logistics can evaluate product labels to ensure compliance. For more detailed information, consult the official NOM documentation and guidelines provided by Mexican regulatory bodies.
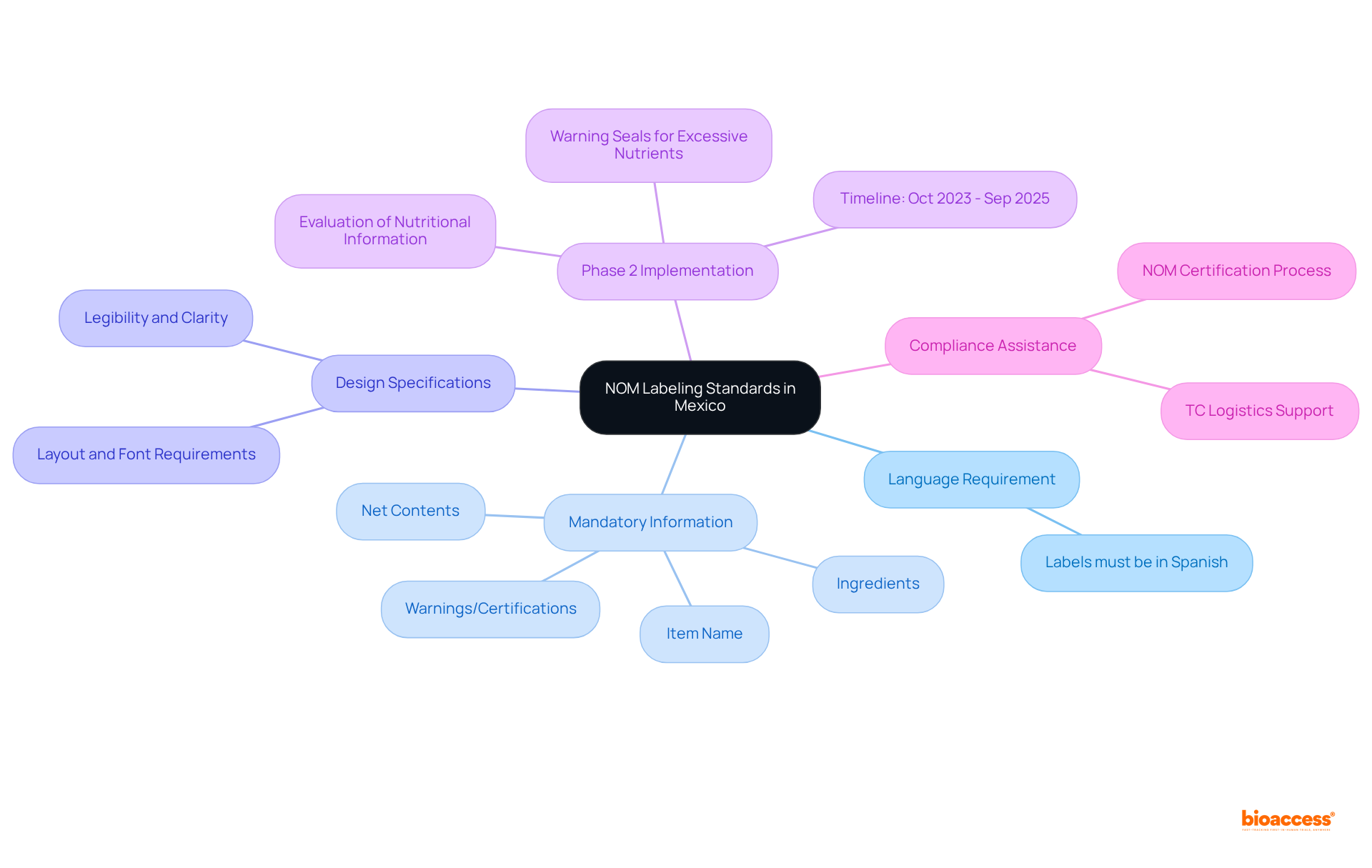
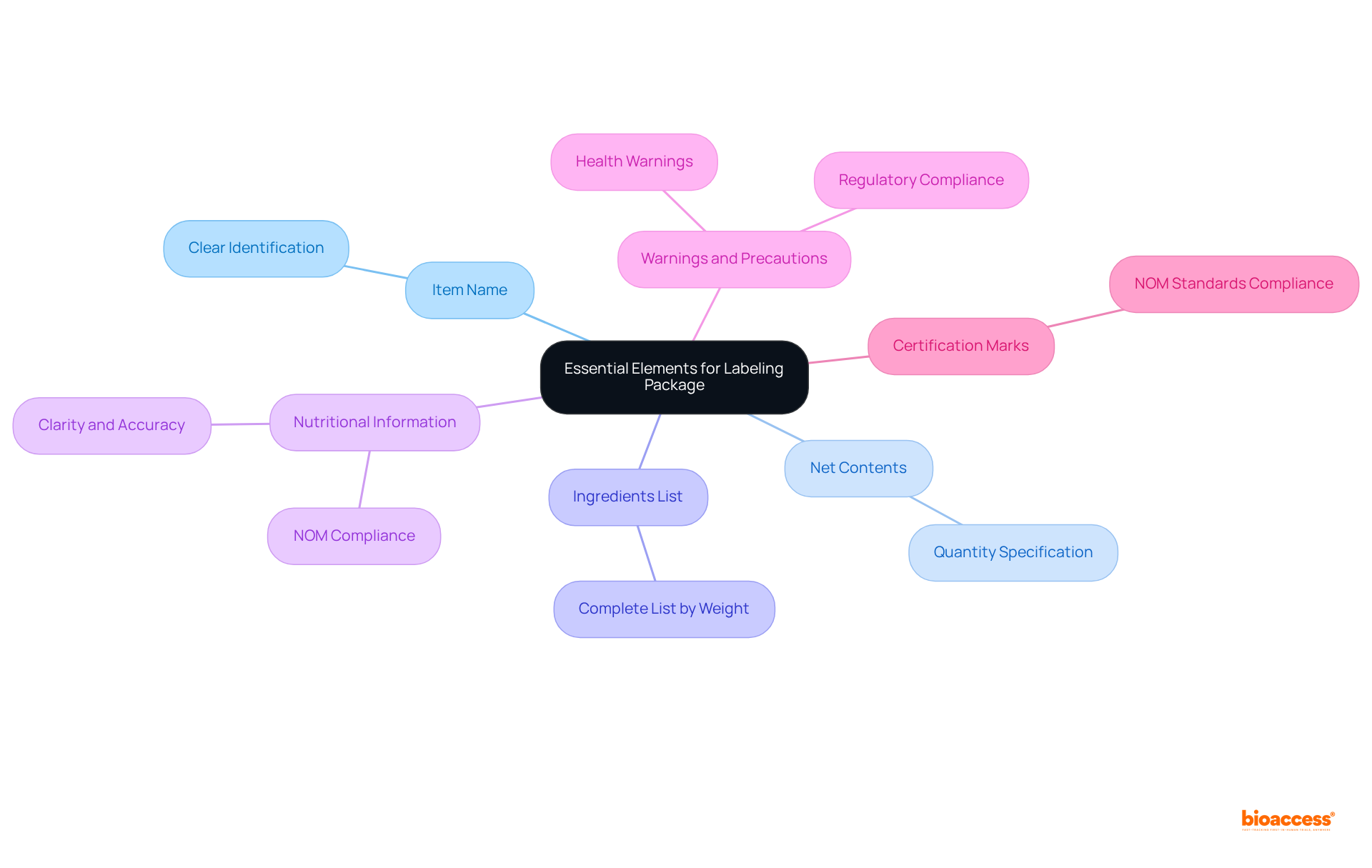
To create a compliant labeling package for medical products in Mexico, it is crucial to include the following essential elements:
In 2025, frequent packaging errors involve leaving out necessary warnings or not displaying the ingredients list accurately. According to Diana Morales-Avilez, "Nutrition information is a public health tool that allows consumers to select healthier foods and beverages." Regulatory specialists highlight that following these guidelines not only guarantees conformity but also boosts consumer trust and product credibility. Successful identification of packages, including the Mexico labeling NOM design package, that meet NOM requirements often includes clear, concise information that aligns with consumer expectations and regulatory standards. Furthermore, the three implementation stages of the Mexican nutrient profile (2020, 2023, and 2025) are essential for grasping the changing regulatory environment and future adherence expectations.
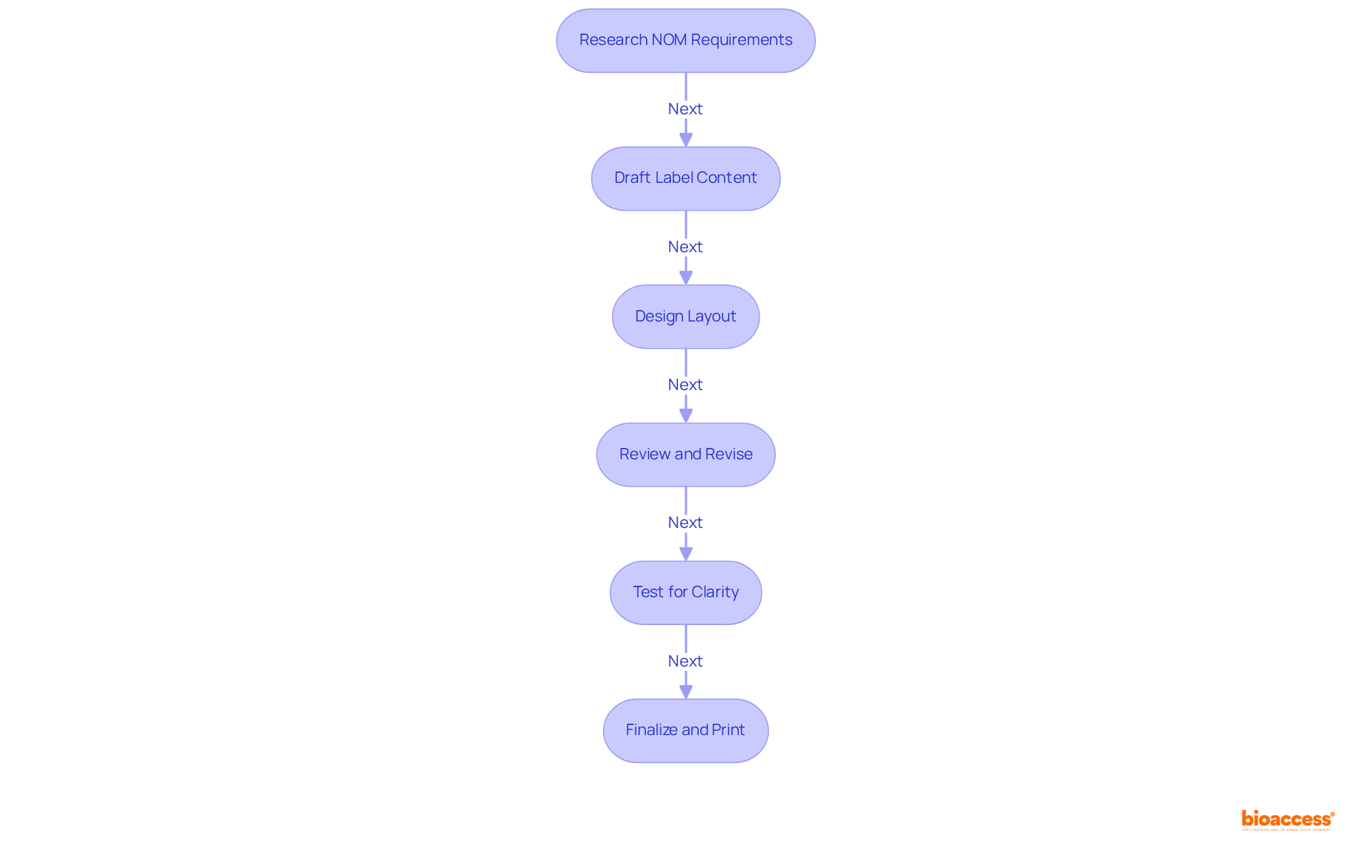
To effectively develop your labeling design package in compliance with NOM standards, it is imperative to follow these essential steps:
Research NOM Requirements: Begin by thoroughly reviewing the latest NOM standards applicable to your specific category, such as NOM-024-SCFI-2013 and NOM-051. Grasping these regulations is essential for adherence, particularly considering the public health issues in Mexico, where 40% of children and teenagers are overweight or obese.
Draft Label Content: Create a comprehensive draft of the label content, ensuring that all mandatory elements, such as product details and nutritional information, are included. This is vital for addressing health concerns and ensuring consumer safety.
Design Layout: Utilize design software to craft a visually appealing layout that adheres to NOM specifications. Prioritize legibility by selecting appropriate fonts and ensuring that warning symbols are prominently displayed, as evidenced in successful case studies during the FOPNL implementation.
Review and Revise: Engage a regulatory expert to review your label. Their perspectives will assist in ensuring that your design fulfills all regulatory standards and avoids potential challenges, emphasizing the significance of professional advice in achieving adherence.
Test for Clarity: Conduct focus groups or surveys to evaluate the clarity and effectiveness of your label design. Collecting feedback from prospective clients can offer valuable insights into how effectively your brand conveys essential information, akin to assessments that demonstrated positive adherence outcomes among firms.
Finalize and Print: After receiving approval, finalize your design and prepare for printing. Ensure that the printing process preserves the quality and integrity of your design, which is crucial for maintaining consumer trust.
By following these steps, you can create a Mexico labeling NOM design package that not only meets NOM standards but also effectively conveys essential product information to consumers, ultimately aiding public health initiatives.
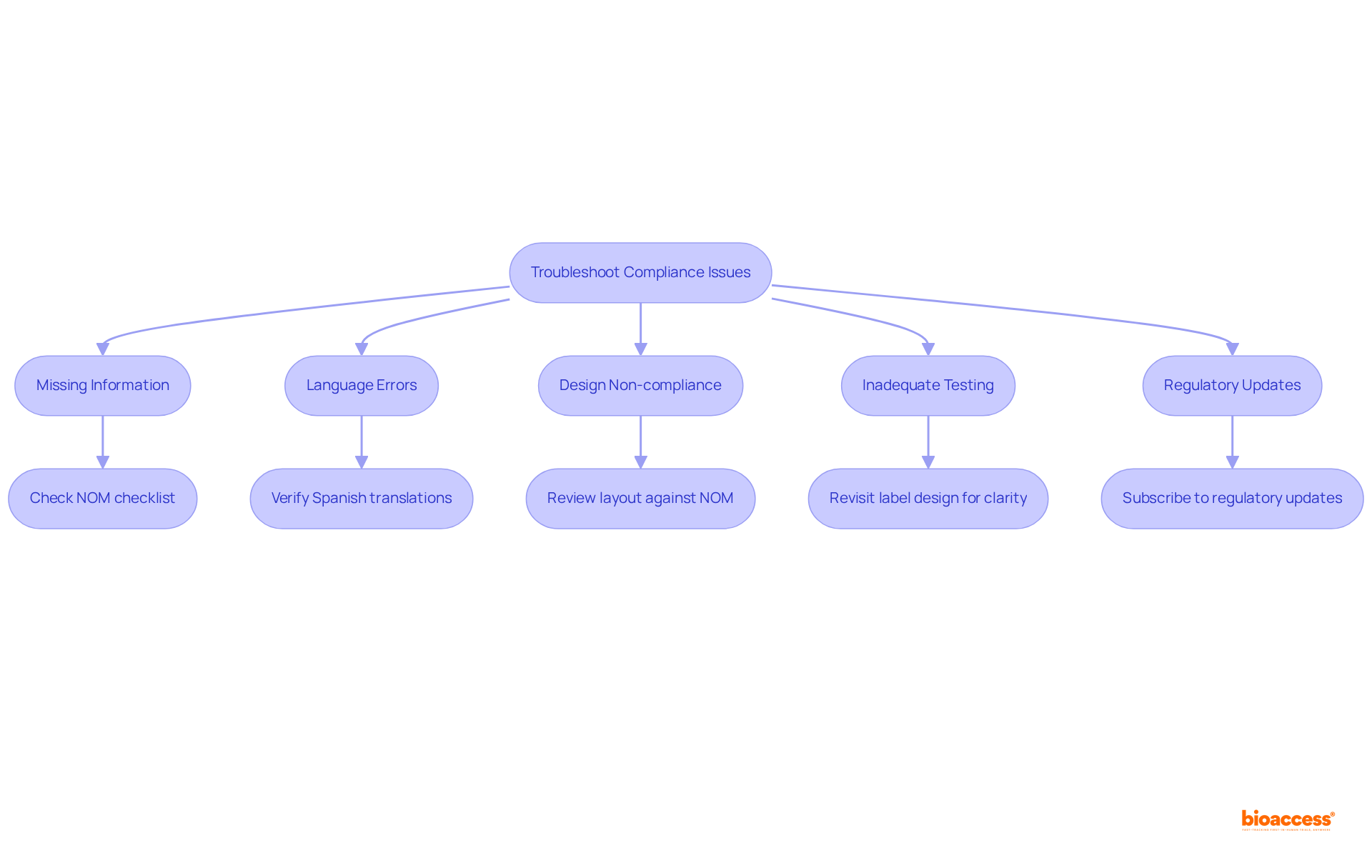
When creating your packaging for identification, it is essential to address several common regulatory concerns effectively. Here’s how to troubleshoot them:
By proactively addressing these issues, you can enhance the adherence and effectiveness of your labeling package, ultimately facilitating a smoother approval process. As Ana Criado, Director of Regulatory Affairs at bioaccess®, points out, "Specialists in regulations offer invaluable experience in ensuring that medical devices meet necessary standards while addressing the complexities of the regulatory landscape." This underscores the importance of thorough adherence in preventing adverse events, as evidenced by the 454,383 device-associated adverse events reported in recent years. Effective compliance not only safeguards patient safety but also facilitates market access in Latin America’s multi-billion dollar healthcare market.
Creating a compliant Mexico labeling NOM design package is essential for businesses aiming to succeed in the Mexican market. Understanding NOM standards is critical for ensuring that product labels provide clear and accurate information to consumers. By adhering to these regulations, companies not only avoid penalties but also foster consumer trust and promote public health.
Key insights discussed include the necessity of including mandatory information such as:
The provided step-by-step guide aids in developing a labeling design package that meets all compliance requirements. Additionally, addressing common compliance issues—like missing information and language errors—can significantly enhance the chances of a smooth approval process.
Ultimately, the significance of adhering to NOM labeling standards extends beyond regulatory compliance; it plays a vital role in safeguarding public health and contributing to informed consumer choices. Businesses are encouraged to prioritize compliance, seek expert advice, and stay updated on regulatory changes to navigate the evolving landscape of product labeling in Mexico effectively. Embracing these practices will not only facilitate market access but also align with broader health initiatives, ensuring a positive impact on the community.
What are NOM labeling standards in Mexico?
The Norma Oficial Mexicana (NOM) standards establish compulsory regulations for the labeling of products sold in Mexico, ensuring that consumers receive accurate and clear information about their purchases.
What language must labels be in according to NOM standards?
All labels must be in Spanish to ensure comprehension by the local population.
What mandatory information must be included on product labels?
Labels are required to include the item name, net contents, ingredients, and any applicable warnings or certifications.
Are there any restrictions on using children's characters on product labels?
Yes, the use of children's characters or appealing elements on products with excessive critical nutrients is prohibited to encourage healthy eating habits.
What are the design specifications for labels under NOM standards?
Labels must comply with specific layout and font requirements to guarantee legibility and clarity.
What is the significance of understanding NOM labeling standards?
Understanding these standards is crucial to avoid compliance issues and potential penalties for non-compliance.
What changes are being implemented in Phase 2 of NOM-051?
As of October 1, 2023, Phase 2 of NOM-051 enhances product information requirements, focusing on the evaluation of complementary nutritional details for prepackaged foods and non-alcoholic beverages.
When will Phase 2 of NOM-051 continue until?
Phase 2 of NOM-051 will continue until September 30, 2025.
How can companies ensure compliance with the new labeling standards?
Companies must review and update their labels to align with the new standards to avoid fines. TC Logistics can assist in evaluating product labels for compliance.
Where can more detailed information about NOM standards be found?
More detailed information can be found in the official NOM documentation and guidelines provided by Mexican regulatory bodies.